Volume 29, Number 9—September 2023
Research
Temporally Associated Invasive Pneumococcal Disease and SARS-CoV-2 Infection, Alaska, USA, 2020–2021
Abstract
Streptococcus pneumoniae can co-infect persons who have viral respiratory tract infections. However, research on S. pneumoniae infections that are temporally associated with SARS-CoV-2 infections is limited. We described the epidemiology and clinical course of patients who had invasive pneumococcal disease (IPD) and temporally associated SARS-CoV-2 infections in Alaska, USA, during January 1, 2020–December 23, 2021. Of 271 patients who had laboratory-confirmed IPD, 55 (20%) had a positive SARS-CoV-2 test result. We observed no major differences in age, race, sex, or underlying medical conditions among IPD patients with and without SARS-CoV-2. However, a larger proportion of IPD patients with SARS-CoV-2 died (16%, n = 9) than for those with IPD alone (4%, n = 9) (p<0.01). IPD patients with SARS-CoV-2 were also more likely to be experiencing homelessness (adjusted OR 3.5; 95% CI 1.7–7.5). Our study highlights the risk for dual infection and ongoing benefits of pneumococcal and COVID-19 vaccination, especially among vulnerable populations.
Invasive pneumococcal disease (IPD) occurs when Streptococcus pneumoniae infects a normally sterile site, such as blood or cerebrospinal fluid. Viral respiratory tract infections caused by rhinovirus, respiratory syncytial virus, and influenza virus are known to predispose patients to secondary bacterial infections, including IPD (1,2). Bacterial infections in patients who have viral respiratory tract infections also have been associated with greater disease severity and increased mortality rates (3–7).
Despite the widespread global circulation of SARS-CoV-2, a limited number of studies have examined IPD and SARS-CoV-2 co-infections. A meta-analysis of case series reported that only 4% of patients who had COVID-19 had a bacterial co-infection and 14% had a bacterial secondary infection (8); no predominant bacterial pathogen was reported. Those bacterial infections occurred mainly among patients in intensive care units. Based on previous studies, only a small proportion of SARS-CoV-2 infections are accompanied by IPD (9,10); however, outcomes of such cases might be more severe (11). A recent national cohort study in England reported that, although substantial nationwide decreases were observed in IPD incidence during the COVID-19 pandemic, persons who had IPD and SARS-CoV-2 co-infections had higher case-fatality rates than did patients who had IPD alone, particularly among older adults (12).
In the United States, Alaska has consistently had among the highest IPD rates in recent years, and a disproportionate burden occurs among Alaska Native persons (13–16). However, the possible interactions between IPD and SARS-CoV-2 infections are unclear. We evaluated and compared the epidemiology and clinical course of patients with IPD with and without temporally associated SARS-CoV-2 infection in Alaska during 2020‒2021.
Data Sources
The Alaska Division of Public Health and the US Centers for Disease Control and Prevention Arctic Investigations Program maintain statewide laboratory-based surveillance for invasive disease caused by S. pneumoniae. Data are collected regarding patient demographic characteristics, clinical syndrome, pneumococcal vaccination, and illness outcomes through medical records. We included patients with IPD in Alaska during January 1, 2020–December 23, 2021. SARS-CoV-2 infection status was determined from nucleic acid amplification test and antigen test results reported to the Alaska Division of Public Health. COVID-19 testing procedures were based on State of Alaska COVID-19 guidance, including testing all symptomatic persons, as well as any asymptomatic person at admission to a healthcare facility. We linked cases by unique patient identifiers. We excluded patients with IPD who had no SARS-CoV-2 testing performed within 30 days before or after their positive S. pneumoniae culture. COVID-19 vaccination status was assigned through linkage with the immunization information system of Alaska.
Definitions
We defined a case of IPD as S. pneumoniae isolated from or bacterial DNA detected in a normally sterile site, including blood, cerebrospinal fluid, pleural fluid, peritoneal fluid, pericardial fluid, joint fluid, bone, or deep tissue, in an Alaska resident. We defined temporally associated SARS-CoV-2 infection as a positive SARS-CoV-2 test result detected on a respiratory specimen collected within 30 days before or after the specimen collection date of the S. pneumoniae culture or positive DNA test result. We defined underlying medical conditions as those conditions specified at the time of IPD reporting that are established risk factors for IPD, including chronic lung disease, cardiovascular disease, immunosuppression, alcoholism, chronic renal disease, current smoking, or diabetes (17). We defined patients as being fully vaccinated against COVID-19 if they had received the second dose of the Pfizer-BioNTech (https://www.pfizer.com) or Moderna (https://www.modernatx.com) mRNA vaccines or 1 dose of the J&J/Janssen (https://www.jnj.com) vaccine >14 days before S. pneumoniae detection. We assigned COVID-19 vaccination status only to IPD patients who were eligible for COVID-19 vaccination at the time of IPD detection, based on the initial COVID-19 vaccine introduction schedule of Alaska. Pneumococcal vaccination was having received >1 dose of 13-valent pneumococcal conjugate vaccine (PCV13) or 23-valent pneumococcal polysaccharide vaccine (PPSV23) >14 days before S. pneumoniae detection. We defined IPD serotype groups as S. pneumoniae serotypes contained in PCV13 plus serotype 6C, those contained in PPSV23 but not in PCV13, and nonvaccine and unknown serotypes.
Statistical Analysis
We compared demographic, epidemiologic, and clinical characteristics of IPD patients with and without a temporally associated SARS-CoV-2 infection. We used χ2 or Fisher exact tests for categorical variables. We performed multivariable logistic regression to identify risk factors for IPD and temporally associated SARS-CoV-2 infection among all patients with IPD. We included known risk factors associated with SARS-CoV-2 infection and retained those that resulted in the best fit models selected by the Akaike Information Criteria through backward stepwise selection. We used R version 4.1.1 (https://www.R-project.org) for all statistical analyses. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (see, e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
During January 2020–December 2021, we identified 330 IPD case-patients. Of those persons, 59 (18%) had no SARS-CoV-2 testing performed within 30 days before or after IPD specimen collection date and were thus excluded from the analysis. Of the excluded persons, 38 (64%) did not undergo SARS-CoV-2 testing because of having a positive culture for S. pneumoniae before COVID-19 testing was initiated in Alaska in March 2020. Other persons might have obtained a positive test result for COVID-19 within the previous 90 days; per testing guidance, these persons were exempted from further testing.
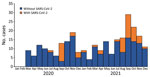
Of the remaining 271 IPD case-patients, 55 (20%) had a temporally associated SARS-CoV-2 infection (Figure). Of those 55 patients, 49 (89%) had a positive SARS-CoV-2 test result on a specimen collected within 30 days before or on the same day as specimen collection for S. pneumoniae detection (Table 1). Only 6 patients had a positive SARS-CoV-2 test result on a specimen collected after their positive test for S. pneumoniae. All IPD patients who died and had a temporally associated SARS-CoV-2 infection (n = 9) had SARS-CoV-2 detected before or concurrent with their IPD; no deaths occurred among patients who had SARS-CoV-2 detected >24 hours after a positive test result for S. pneumoniae.
Seven (3%) IPD cases were reported among patients <20 years of age (Table 2), including 1 patient who had a temporally associated SARS-CoV-2 infection (detected 9 days before IPD). The remaining 264 (97%) patients who had IPD were adults >20 years of age (median age 52 years, range 20–88 years); 54 had a temporally associated SARS-CoV-2 infection. No major differences in sex, age, race, region, or underlying medical conditions were reported among IPD patients with and without temporally associated SARS-CoV-2 infection (Table 2). A total of 19 (35%) of 55 patients who had IPD and temporally associated SARS-CoV-2 infections were persons experiencing homelessness, compared with 39 (18%) of 216 patients with IPD alone (p = 0.01). IPD cases with temporally associated SARS-CoV-2 infection were more likely to occur during July‒September 2021, the period when COVID-19 hospitalizations peaked in Alaska (Table 2) (18).
Patients who had IPD and temporally associated SARS-CoV-2 infection were more likely to show a clinical syndrome of pneumonia defined in the patient’s medical records, but we found no differences in rates of hospitalization between patients with or without temporally associated SARS-CoV-2 infection. Among 55 IPD patients who had temporally associated SARS-CoV-2 infection, 9 (16%) died, compared with 9 (4%) of 216 patients who had IPD alone (p<0.01).
Of 271 patients who had IPD, 133 (49%) had received >1 dose of pneumococcal vaccine; no difference was reported in pneumococcal vaccination coverage rates between patients with or without temporally associated SARS-CoV-2 infection. Of 139 patients who had IPD and were eligible for COVID-19 vaccination, only 52 (37%) had completed a COVID-19 vaccine primary series. No difference in COVID-19 vaccination rates was reported between patients with or without temporally associated SARS-CoV-2 infection.
Overall, 30 (55%) of the IPD cases among patients who had SARS-CoV-2 infections were caused by S. pneumoniae serotypes contained in the PCV13 vaccine, compared with 167 (63%) of the IPD cases in patients without SARS-CoV-2 infection (Table 3). Serotype 4 was the most common cause of IPD among both groups, including 44% (24/55) of IPD cases with SARS-CoV-2 infection and 56% (120/216) of IPD cases without SARS-CoV-2. In contrast, a higher proportion of patients who had temporally associated SARS-CoV-2 infection had IPD attributable to a serotype contained in PPSV23 but not PCV13 or a serotype that is not in either vaccine. Multivariable analysis showed that, among patients who had IPD, persons experiencing homelessness were more likely to have a temporally associated SARS-CoV-2 infection than persons not experiencing homelessness (adjusted odds ratio 3.5, 95% CI 1.7–7.5) (Table 4).
We found that, among 271 patients who had laboratory confirmed IPD, 55 (20%) also had a temporally associated SARS-CoV-2 infection. For most of those patients, SARS-CoV-2 infection was detected before or concurrent with IPD. This finding is similar to what has been observed for other viral infections, such as influenza viruses, rhinoviruses, and adenoviruses (19–22). The mechanism through which SARS-CoV-2 infection might predispose a person to IPD is unclear. However, as for other viral infections, the cause is likely multifactorial, including epithelial damage, changes in airway function, upregulation and exposure of receptors, inhibited immune response, or enhancement of inflammation (23–26).
We found that IPD patients who died were more likely to have a temporally associated SARS-CoV-2 infection. All patients who died and had IPD and a temporally associated SARS-CoV-2 infection also had their infection detected either before or concurrently with the IPD. This finding suggests secondary bacterial infection as a possible complicating factor for death. All deceased patients with a SARS-CoV-2 infection had COVID-19 listed as a cause of death. However, because of a lack of detailed clinical data and similar clinical manifestations of both diseases, we were unable to determine how IPD, SARS-CoV-2 infection, or a combination of both contributed to the patients’ deaths.
Among all reported patients who had IPD, persons experiencing homelessness were more likely to have a temporally associated SARS-CoV-2 infection. People experiencing homelessness are known to be at increased risk for IPD, probably attributable to staying in congregate settings, limited uptake of routine vaccinations, and higher prevalence of predisposing medical conditions (27–31). However, the possibility also exists that our findings were caused by increased detection of SARS-CoV-2 infection from routine screening in homeless shelters.
The first limitation of our study was that we were not able to obtain symptom onset dates for IPD or SARS-CoV-2 infection, indicating that timing of infection might differ from timing of detection in our results. Second, we were unable to determine how many SARS-CoV-2 tests persons experiencing homelessness received during the study period relative to the general population. Therefore, we could not calculate whether increased SARS-CoV-2 testing contributed to the observed increased odds of SARS-CoV-2 infection in persons experiencing homelessness. Third, our study was limited in assessing the interaction between homelessness and death because of the small number of deaths among persons experiencing homelessness. However, it is useful to recognize that homelessness itself has consistently been associated as an independent risk factor for increased deaths. Fourth, we were unable to obtain detailed clinical information regarding those patients who died and had IPD and a temporally associated SARS-CoV-2 infection, which indicates that we could not determine the etiologic role that SARS-CoV-2 had in their death. We also assigned COVID-19 vaccination eligibility based on the phased rollout in the Alaska general population. Certain IPD patients might have been eligible before this date on the basis of immunocompromising conditions and occupational risk factors (e.g., healthcare workers) and underestimated the number of eligible persons not vaccinated. We also did not examine risk factors for IPD and deaths because the total number of patients who died was small (n = 18) probably resulting in small sample bias from maximum-likelihood estimation in multivariable models. Fifth, because we only included patients who had IPD, we cannot infer the association concerning other noninvasive pneumococcal infections with SARS-CoV-2. Nevertheless, the study has multiple strengths, including linkage of statewide data sources to effectively capture all reported cases of IPD and COVID-19 in the state during the study period and a robust epidemiologic comparison of persons co-infected with IPD and SARS-CoV-2 to those infected with IPD alone.
In conclusion, we found that ≈1 of 5 patients who had IPD in Alaska during 2020–2021 had a temporally associated SARS-CoV-2 infection, and a greater proportion of patients who had IPD and temporally associated SARS-CoV-2 infection died compared with persons who had IPD alone. Persons experiencing homelessness who had IPD were at increased risk for temporally associated SARS-CoV-2 infection. Healthcare providers should be aware of the added risks associated with dual infection and the ongoing benefits of pneumococcal and COVID-19 vaccination, especially among vulnerable populations (17,32,33).
Dr. Newell is an epidemic intelligences service officer in the Center for Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, Atlanta, GA, assigned to the Alaska Division of Public Health. Her primary research interests focus on the epidemiology of infectious diseases.
References
- Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. DOIPubMedGoogle Scholar
- Metersky ML, Masterton RG, Lode H, File TM Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16:e321–31. DOIPubMedGoogle Scholar
- Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, Deleon-Carnes M, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205:895–905. DOIPubMedGoogle Scholar
- Martín-Loeches I, Sanchez-Corral A, Diaz E, Granada RM, Zaragoza R, Villavicencio C, et al.; H1N1 SEMICYUC Working Group. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest. 2011;139:555–62. DOIPubMedGoogle Scholar
- Kneyber MC, Blussé van Oud-Alblas H, van Vliet M, Uiterwaal CS, Kimpen JL, van Vught AJ. Concurrent bacterial infection and prolonged mechanical ventilation in infants with respiratory syncytial virus lower respiratory tract disease. Intensive Care Med. 2005;31:680–5. DOIPubMedGoogle Scholar
- Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–8. DOIPubMedGoogle Scholar
- Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–51. DOIPubMedGoogle Scholar
- Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–9. DOIPubMedGoogle Scholar
- Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–75. DOIPubMedGoogle Scholar
- Suzuki M, Hayakawa K, Asai Y, Terada M, Kitajima K, Tsuzuki S, et al. Characteristics of hospitalized COVID-19 patients with other respiratory pathogens identified by rapid diagnostic test. J Infect Chemother. 2023;29:539–45. DOIPubMedGoogle Scholar
- Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS One. 2021;16:
e0251170 . DOIPubMedGoogle Scholar - Amin-Chowdhury Z, Aiano F, Mensah A, Sheppard CL, Litt D, Fry NK, et al. Impact of the COVID-19 pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with SARS-CoV-2: prospective national cohort study, England. Clin Infect Dis. 2021;72:e65–75. DOIPubMedGoogle Scholar
- Massay S, Bobo M, Stewart P, Fischer M, Bressler S, Bruden D, et al. Updated adult pneumococcal vaccination recommendations. Epi Bulletin 2022;8 [cited 2023 Jul 20]. http://www.epi.alaska.gov/bulletins/docs/b2022_08.pdf
- Bruce MG, Singleton R, Bulkow L, Rudolph K, Zulz T, Gounder P, et al. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine. 2015;33:4813–9. DOIPubMedGoogle Scholar
- Singleton R, Wenger J, Klejka JA, Bulkow LR, Thompson A, Sarkozy D, et al. The 13-valent pneumococcal conjugate vaccine for invasive pneumococcal disease in Alaska native children: results of a clinical trial. Pediatr Infect Dis J. 2013;32:257–63. DOIPubMedGoogle Scholar
- Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29:251–6. DOIPubMedGoogle Scholar
- Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner AJ, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109–17. DOIPubMedGoogle Scholar
- Alaska Division of Public Health. Alaska State COVID-19 cases dashboard [cited 2023 Jul 27]. https://experience.arcgis.com/experience/af2efc8bffbf4cdc83c2d1a134354074.
- O’Brien KL, Walters MI, Sellman J, Quinlisk P, Regnery H, Schwartz B, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–9. DOIPubMedGoogle Scholar
- Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–12. DOIPubMedGoogle Scholar
- Peltola V, Heikkinen T, Ruuskanen O, Jartti T, Hovi T, Kilpi T, et al. Temporal association between rhinovirus circulation in the community and invasive pneumococcal disease in children. Pediatr Infect Dis J. 2011;30:456–61. DOIPubMedGoogle Scholar
- Håkansson A, Kidd A, Wadell G, Sabharwal H, Svanborg C. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun. 1994;62:2707–14. DOIPubMedGoogle Scholar
- Deinhardt-Emmer S, Böttcher S, Häring C, Giebeler L, Henke A, Zell R, et al. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J Virol. 2021;95:e00110–21. DOIPubMedGoogle Scholar
- Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 20201;5:eabd1554.
- Pallikkuth S, Williams E, Pahwa R, Hoffer M, Pahwa S. Association of Flu specific and SARS-CoV-2 specific CD4 T cell responses in SARS-CoV-2 infected asymptomatic heath care workers. Vaccine. 2021;39:6019–24. DOIPubMedGoogle Scholar
- Hoepel W, Chen HJ, Geyer CE, Allahverdiyeva S, Manz XD, de Taeye SW, et al. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med. 2021;13:
eabf8654 . DOIPubMedGoogle Scholar - Mosites E, Zulz T, Bruden D, Nolen L, Frick A, Castrodale L, et al. Risk for invasive streptococcal infections among adults experiencing homelessness, Anchorage, Alaska, USA, 2002–2015. Emerg Infect Dis. 2019;25:1911–8. DOIPubMedGoogle Scholar
- Steinberg J, Bressler SS, Thompson GC. Invasive pneumococcal disease among adults experiencing homelessness in Anchorage, Alaska 2005–2020. Presented at: 12th International Symposium on Pneumococci and Pneumococcal Diseases; Toronto, Ontario Canada; June 19–23, 2022.
- Lemay J-A, Ricketson LJ, Zwicker L, Kellner JD. Homelessness in adults with invasive pneumococcal disease in Calgary, Canada. Open Forum Infect Dis. 2019;6:
ofz362 . DOIPubMedGoogle Scholar - McKee G, Choi A, Madill C, Marriott J, Kibsey P, Hoyano D II. Outbreak of invasive Streptococcus pneumoniae among an inner-city population in Victoria, British Columbia, 2016-2017. Can Commun Dis Rep. 2018;44:317–22. DOIPubMedGoogle Scholar
- Beall B, Walker H, Tran T, Li Z, Varghese J, McGee L, et al. Upsurge of conjugate vaccine serotype 4 invasive pneumococcal disease clusters among adults experiencing homelessness in California, Colorado, and New Mexico. J Infect Dis. 2021;223:1241–9. DOIPubMedGoogle Scholar
- Kobayashi M, Farrar JL, Gierke R, Leidner AJ, Campos-Outcalt D, Morgan RL, et al.; ACIP Pneumococcal Vaccines Work Group; CDC Contributors. CDC Contributors. Use of 15-valent pneumococcal conjugate vaccine among U.S. children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174–81. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Overview of COVID-19 vaccines [cited 2023 Jul 20]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html.
Figure
Tables
Cite This ArticleOriginal Publication Date: July 28, 2023
Table of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Katherine Newell, Centers for Disease Control and Prevention, 3601 C St, Ste 540, Anchorage AK, USA
Top