Volume 29, Number 9—September 2023
Dispatch
Rat Hepatitis E Virus in Norway Rats, Ontario, Canada, 2018–2021
Abstract
We tested liver samples from 372 Norway rats (Rattus norvegicus) from southern Ontario, Canada, during 2018–2021 to investigate presence of hepatitis E virus infection. Overall, 21 (5.6%) rats tested positive for the virus. Sequence analysis demonstrated all infections to be rat hepatitis E virus (Rocahepevirus ratti genotype C1).
Hepatitis E virus (HEV) is a nonenveloped, single-stranded, positive-sense RNA virus within the family Hepeviridae, subfamily Orthohepevirinae, which is divided into 4 genera: Paslahepevirus, Rocahepevirus, Chirohepevirus, and Avihepevirus (1). Human hepatitis E is primarily caused by Paslahepevirus balayani (genotypes 1–4), and infection generally causes an acute, self-limiting disease, but severe and chronic hepatitis and extrahepatic manifestations can occur in immunocompromised patients (2). Paslahepevirus balayani genotypes 1 and 2 are endemic in developing countries, circulating in humans and transmitted primarily via the fecal-oral route through contaminated drinking water. Sporadic cases of hepatitis E infection caused by zoonotic transmission of HEV (Paslahepevirus balayani genotypes 3 and 4) are increasingly reported in industrialized countries (3). Infections are acquired through direct contact with infected animals, environmental contamination with animal feces, and foodborne transmission from eating undercooked pork, venison, and wild boar meat (3).
Additional HEV variants have been reported in a diversity of animal species, and zoonotic transmission from animal reservoirs is a growing public health concern. Norway rats (Rattus norvegicus) have been shown to carry swine HEV (Paslahepevirus balayani genotype 3) and are natural reservoirs of HEV variants within the species Rocahepevirus ratti genotype C1 (rat HEV) (4). Since it was first detected in Germany in 2010, rat HEV has been identified in Norway rats from the United States, China, Vietnam, and 13 countries in Europe (5). Recently, cases of acute hepatitis caused by rat HEV have been reported in Hong Kong, Canada (infection acquired in Central Africa), and Spain (6–8). Those reports raise concerns regarding the potential risk for rat HEV transmission to humans and hepatitis E as an emerging infectious disease worldwide.
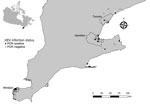
We conducted a study to investigate HEV infection in Norway rats from southern Ontario, Canada, and identify associations between host factors, season, land use, and year of collection. We obtained rat carcasses through collaboration with pest control professionals working in southern Ontario. Our rat and sample collection methods have been previously described and evaluated as a source of samples for zoonotic pathogen surveillance (9). We studied 372 Norway rats (species determined by external morphology) from 161 unique geographic coordinates within southern Ontario during November 2018–June 2021 (Figure 1). During necropsy, we recorded rat demographic characteristic data (Table) and collected liver samples aseptically. Most rats in our sample were sexually mature (65%), and there were more females (51.2%) than males (48.8%). We noted the body condition of rats to be poor (emaciated or underconditioned) in 69.1% and good (well conditioned or overconditioned) in 30.9%. We could not determine sex (3%), sexual maturity (3.2%), or body condition (2.4%) in a minority of rats because of poor carcass condition. We categorized rats by collection location as residential (52.4%), industrial (17.5%), institutional (15.6%), commercial (8.9%), and mixed (5.6%) land use. We collected most rats during the winter (36.8%) and fall (36.8%), followed by spring (21.8%) and summer (4.6%). To account for low sample size in the summer, we recategorized seasonal data as summer/fall (June–November) and winter/spring (December–May).
We screened liver RNA extracts for the presence of HEV by real-time PCR by using previously described primers and probes (10). Of 372 rats tested, 21 (5.6%, 95% CI 3.5%–8.5%) rats from 16 distinct locations in 7 cities/towns were positive for HEV (Figure 1). The odds of HEV infection were significantly higher in sexually mature rats (odds ratio 3.99, 95% CI 1.14–21.47; p = 0.025). By using exact logistic regression models, we observed no association with sex, body condition, land use, season, or year of collection (Table).
We amplified positive samples by using a previously described heminested PCR to generate an amplicon from the open reading frame (ORF) 1 region (11). We retrieved a 283-nt fragment of ORF1 from 17 samples and analyzed generated sequences with Lasergene software (DNASTAR, https://www.dnastar.com). We did not obtain sequence data for 4 rats. We aligned sequences with select GenBank reference sequences representing HEV genotypes currently known to infect rats, as well as rat HEV found in humans. Phylogenetic analysis of the partial ORF1-derived sequences showed that all PCR amplicons were rat HEV. We grouped rat HEV sequences from southern Ontario (GenBank accession numbers OQ617169–85) into 4 distinct clusters (Figure 2), with relatively low genetic divergence (14%). Sequences in our study had the highest nucleotide homology with rat HEV sequences from rats in the United States (83.3%), followed by Germany (82.2%), Vietnam (71.5%), and Indonesia (71.3%). Southern Ontario shares a border with 2 US states, New York and Michigan, and the westernmost samples from Windsor were collected directly adjacent to Detroit, Michigan. We noted Ontario rat HEV sequences to be genetically distinct (24.6% divergence) from rat HEV sequences reported in humans.
Laboratory analysis of samples taken from Norway rats in southern Ontario, Canada, revealed hepatitis E virus RNA in 21 (5.6%, 95% CI 3.5%–8.5%) of 372 rats, and phylogenetic analysis demonstrated that these sequences were closely related to those found in rats from other countries. Detection of rat HEV (R. ratti genotype C1) in Norway rats in our study shows that this virus is broadly distributed within southern Ontario, including 3 major cities (i.e., Toronto, Hamilton, and Windsor), and may be endemic in Norway rat populations. An absence of PCR-positive rats in some areas of southern Ontario may be the result of undersampling rather than an indication that HEV is absent in these populations.
We observed that sexually mature rats were at significantly greater odds of being infected with HEV than immature rats. This observation is in contrast to findings from previous studies of rats, which found no association with age and infection status (14,15). We concede that this disparity in findings might be owing to methodological differences in how age classes were defined (i.e., sexual maturity [open vaginal orifice in females, scrotal testes in males] vs. weight). The observed association in our study might be the result of cumulative exposure to HEV leading to increased risk for infection over time and behaviors in sexually mature rats that may increase transmission (e.g., exploratory and aggressive behaviors).
To date, 12 human cases of rat HEV have been reported in Hong Kong, Canada, and Spain (6–8). Although zoonotic transmission from rats to humans has been suggested, the exact source and route of transmission in these cases remains unclear. Notably, human hepatitis E caused by rat HEV may be underreported because of subclinical or mild infection, limited awareness, and diagnostic testing techniques for HEV that might not detect rat HEV. Further studies are needed to investigate potential modes and patterns of transmission and elucidate the zoonotic potential of rat HEV and associated public health risks.
This report of rat HEV (R. ratti genotype C1) in Canada provides further evidence that this virus has a broad geographic distribution globally and may be endemic in Norway rats. Our study highlights the importance of continued surveillance for HEV in rats and the need for additional research regarding the role of rats in human hepatitis E.
Dr. Robinson is a postdoctoral researcher in the Department of Pathobiology, Ontario Veterinary College, University of Guelph, Ontario, Canada. Her research interests include the ecology and epidemiology of zoonotic pathogens associated with rats and other wildlife.
Acknowledgments
We thank Leonard Shirose, Brian Stevens, Laura Dougherty, Rachel Finer, and Simon Jeeves for their support and assistance with sample processing. We greatly appreciate Windsor Pest Control (Catherine Trudell), Abell Pest Control, and Orkin Canada for submitting carcasses.
Funding was provided by the Natural Science and Engineering Research Council. S.J.R. was supported by an Ontario Veterinary College PhD Fellowship and an Ontario Graduate Scholarship.
References
- Purdy MA, Drexler JF, Meng XJ, Norder H, Okamoto H, Van der Poel WHM, et al. ICTV virus taxonomy profile: Hepeviridae 2022. J Gen Virol. 2022;103:103. DOIPubMedGoogle Scholar
- Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082–95. DOIPubMedGoogle Scholar
- Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. DOIPubMedGoogle Scholar
- Kenney SP. The current host range of hepatitis E viruses. Viruses. 2019;11:452. DOIPubMedGoogle Scholar
- Wang B, Harms D, Yang XL, Bock CT. Orthohepevirus C: an expanding species of emerging hepatitis E virus variants. Pathogens. 2020;9:154. DOIPubMedGoogle Scholar
- Sridhar S, Yip CCY, Wu S, Chew NFS, Leung KH, Chan JFW, et al. Transmission of rat hepatitis E virus infection to humans in Hong Kong: A clinical and epidemiological analysis. Hepatology. 2021;73:10–22. DOIPubMedGoogle Scholar
- Andonov A, Robbins M, Borlang J, Cao J, Hatchette T, Stueck A, et al. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J Infect Dis. 2019;220:951–5. DOIPubMedGoogle Scholar
- Rivero-Juarez A, Frias M, Perez AB, Pineda JA, Reina G, Fuentes-Lopez A, et al.; HEPAVIR and GEHEP-014 Study Groups. Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. J Hepatol. 2022;77:326–31. DOIPubMedGoogle Scholar
- Robinson SJ, Finer R, Himsworth CG, Pearl DL, Rousseau J, Weese JS, et al. Evaluating the utility of pest control sourced rats for zoonotic pathogen surveillance. Zoonoses Public Health. 2022;69:468–74. DOIPubMedGoogle Scholar
- Mulyanto, Suparyatmo JB, Andayani IGAS, Khalid, Takahashi M, Ohnishi H, et al. Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Research. 2014;179:102–12.
- Drexler JF, Seelen A, Corman VM, Fumie Tateno A, Cottontail V, Melim Zerbinati R, et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J Virol. 2012;86:9134–47. DOIPubMedGoogle Scholar
- Lefort V, Longueville JE, Gascuel O. SMS: smart model selection in PhyML. Mol Biol Evol. 2017;34:2422–4. DOIPubMedGoogle Scholar
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–52. DOIPubMedGoogle Scholar
- Murphy EG, Williams NJ, Jennings D, Chantrey J, Verin R, Grierson S, et al. First detection of Hepatitis E virus (Orthohepevirus C) in wild brown rats (Rattus norvegicus) from Great Britain. Zoonoses Public Health. 2019;66:686–94. DOIPubMedGoogle Scholar
- Ryll R, Bernstein S, Heuser E, Schlegel M, Dremsek P, Zumpe M, et al. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet Microbiol. 2017;208:58–68. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: August 08, 2023
Table of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sarah J. Robinson, Department of Pathobiology, Ontario Veterinary College, University of Guelph, 50 Stone Rd East, Guelph, ON N1G 2W1, Canada
Top