Volume 29, Number 9—September 2023
Dispatch
Anaplasma bovis–Like Infections in Humans, United States, 2015–2017
Abstract
We detected the DNA of an Anaplasma bovis–like bacterium in blood specimens from 4 patients from the United States with suspected tickborne illnesses. Initial molecular characterization of this novel agent reveals identity to A. bovis–like bacteria detected in Dermacentor variabilis ticks collected from multiple US states.
The genus Anaplasma includes several species of tickborne, zoonotic pathogens of global importance. Three recognized species (Anaplasma phagocytophilum, Anaplasma ovis, and Anaplasma bovis) and one provisionally named species (Anaplasma capra) are associated with moderately severe to severe disease in humans (1). Human infections with A. bovis, a pathogen first identified in monocytes of cattle in Algeria in 1936 and subsequently detected in other countries in Africa, Asia, and the Americas, were reported from China in 2017 (1–3). In 2015, a targeted metagenomic approach designed to amplify the V1–V2 region of the bacterial 16S rRNA (rrs) gene identified DNA of an A. bovis–like agent in blood specimens from 2 US patients with suspected tickborne illnesses (4). The agent demonstrated 100% identity across a 357-bp region of rrs to A. bovis–like sequences amplified from several human-biting Dermacentor tick species in North America (4). An additional 2 US patients positive for this same Anaplasma species were identified in 2017 (L. Kingry et al., unpub. data), although the genetic identity of this pathogen remained limited to the same 357-bp sequence of rrs (5–7). To further characterize the phylogenetic position of this novel agent, we evaluated additional sequences to determine the uniqueness of this strain among the expanding global complex of A. bovis–like bacteria.
We extracted DNA from 100 µL of EDTA-treated whole blood obtained from 4 patients from whom partial rrs sequences of an A. bovis–like agent were identified from a targeted metagenomics assessment of whole blood specimens collected from US patients with suspected tickborne disease (4; L. Kingry et al., unpub. data). DNA extracts containing A. bovis DNA were also available from an adult Dermacentor andersoni tick collected in Saskatchewan Landing Provincial Park in Saskatchewan, Canada, and from 5 adult Dermacentor variabilis ticks collected in Washita County, Oklahoma; Floyd County, Iowa; and Sarpy and Cass Counties, Nebraska, from which partial rrs sequences most similar with A. bovis were amplified previously (5,6; L. Kingry).
We amplified segments of the rrs, citrate synthase (gltA), and heat shock chaperon (groEL) genes using Taq PCR Master Mix Kit (QIAGEN, https://www.qiagen.com) (Table 1). Each 20-µL primary reaction consisted of 1 µM of each primer, 10 µL Taq Master Mix, 2 µL DNA, and 6 µL molecular-grade water. Secondary reactions (groEL only) consisted of 1 µM of each primer, 10 µL Taq Master Mix, 1 µL primary PCR product, and 7 µL molecular-grade water. We resolved PCR amplicons on a 1% agarose gel in Tris-acetate-EDTA buffer and cut amplicons from the gel and purified using a Wizard SV Gel and PCR Clean-up kit (Promega, https://www.promega.com). We sequenced each purified amplicon (1 µL) bidirectionally using a Big Dye Terminator v3.1 Cycle Sequencing Kit, purified using a BigDye XTerminator Purification Kit, and sequenced using an ABI 3500 Genetic Analyzer (all from ThermoFisher Scientific, https://www.thermofisher.com).
We used Geneious Prime version 2021.0.3 (https://www.geneious.com) to assemble and align consensus sequences and infer the phylogenetic relationships between DNA sequences (12). Only 3 sources of genetic information for A. bovis were available in GenBank that provided complete or partial sequence data at all 3 loci, including those amplified from the blood of a raccoon (Procyon lotor) captured in Hokkaido, Japan (13); a goat (Capra sp.) from Shaanxi Province, China; and a cow (Bos taurus) from Shaanxi Province, China. The rrs, gltA, and groEL nucleotide sequences amplified from the human samples were submitted to GenBank and assigned the accession numbers OQ693620 (rrs), OQ694770 (gltA), and OQ693619 (groEL).
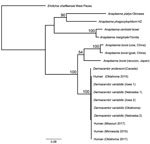
The rrs sequences (599-bp) of the 4 human samples were 100% identical to each other and to those amplified from a D. andersoni tick and 5 D. variabilis ticks; the sequences also showed 98.3% identity to the rrs sequences amplified from blood specimens obtained from the cow from China, 98% to those from the goat from China, and 97.8% identity to those from the raccoon from Japan. The 826-bp gltA sequences from the 4 human samples were 100% identical to each other and to all sequences from D. variabilis ticks; they also were 99.4% identical to the 827-bp sequence from the D. andersoni tick. When trimmed to 356 bp to match the sequence lengths available in GenBank of those from the cow and goat from China, the North America sequences amplified from humans and ticks shared only 78.6%–79.4% identity with the sequences from China. The groEL sequences (1,079-bp) of the human samples were 100% identical to each other and to the corresponding sequences amplified from all 5 D. variabilis ticks and showed 99.4% identity to the groEL sequence amplified from the D. andersoni tick. Those samples showed only 85.4% identity to the A. bovis sequences from the raccoon from Japan and 84.6% identity to the sequences from the cow and goat from China. Phylogenetic analyses using concatenated sequences from the 3 loci produced an inferred consensus tree that grouped human and North America Dermacentor spp. tick samples with the other A. bovis sequences but with strong statistical support (100%) for the separation of A. bovis–like sequences from North America and those from China and Japan (Figure).
A novel and presumably tickborne pathogen of humans was identified in blood of patients from the central and upper midwestern United States during 2015–2017 (Table 2). The amplification of a thus far genetically identical agent from D. variabilis ticks suggests that this tick species could represent a vector of this A. bovis–like agent in the United States. This bacterium is also related to a worldwide complex of bacteria, detected in multiple species of ticks and domesticated and wild animals, designated collectively as A. bovis. Because A. bovis has never been cultured in vitro, neither a type strain nor a complete genome exist for this pathogen. Only 3 genetic loci from A. bovis exist in GenBank, and few sources provide complete sequences for all loci from the same sample. As seen in this evaluation, the level of nucleotide identity among samples can vary considerably at an individual locus and hamper efforts to establish genetic relatedness of A. bovis–like bacteria.
The spectrum of disease and epidemiology associated with human infections caused by this novel A. bovis–like agent remains unknown. Presumably, human infections with this agent in the United States are uncommon, because this bacterium was detected only 4 times from 29,928 residual clinical samples obtained during 2014–2019. By comparison, 1,236 infections with A. phagocytophilum and 345 infections with Ehrlichia spp. were identified from this investigation during the same period (5; L. Kingry et al., unpub. data). The study design that enabled the discovery of this novel agent also precluded the collection of clinical details of infected patients; nonetheless, an A. bovis–like pathogen was detected recently in blood of patients from Anhui and Jiangxi Provinces in China who had illnesses characterized predominantly by fever, myalgia, fatigue, anorexia, and thrombocytopenia (3). In the United States, A. bovis–like bacteria have been detected in blood samples from cottontail rabbits (Sylvilagus spp.) from Massachusetts, Georgia, and Texas and from black-tailed jackrabbits (Lepus californicus) from Texas (14,15). Developing a specific molecular assay could help identify additional patients infected with this novel agent and clarify the tick and wildlife species involved in its natural history and transmission to humans.
Dr. Karpathy is a microbiologist for the Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. His research interests include the genetic characterization and genomics of rickettsial pathogens.
Acknowledgments
We thank Paula Lado, Michelle Allerdice, Joy Hecht, and Maria F.B.M. Galletti for providing DNA from the D. variabilis tick samples used in the molecular evaluations. We also thank the TickNet AMD Emerging Infections Program Team at the Minnesota Department of Health, the Mayo Clinic, and Bacterial Diseases Branch in the Division of Vector-Borne Diseases at the Centers for Disease Control and Prevention for assistance with the identification of the human cases.
S.J.D. is an employee of the Government of Canada (His Majesty the King in Right of Canada, 2023).
References
- Li H, Zheng Y-C, Ma L, Jia N, Jiang B-G, Jiang R-R, et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis. 2015;15:663–70. DOIPubMedGoogle Scholar
- Donatien A, Lestoquard F. Rickettsiose bovine Algerienne a R. bovis. Bull Soc Pathol Exot. 1940;33:245–8.
- Lu M, Chen Q, Qin X, Lyu Y, Teng Z, Li K, et al. Anaplasma bovis infection in fever and thrombocytopenia patients—Anhui Province, China 2021. China CDC Wkly. 2022;4:249–53. DOIPubMedGoogle Scholar
- Kingry L, Sheldon S, Oatman S, Pritt B, Anacker M, Bjork J, et al. Targeted metagenomics for clinical detection and discovery of bacterial tick-borne pathogens. J Clin Microbiol. 2020;58:e00147–20. DOIPubMedGoogle Scholar
- Chilton NB, Dergousoff SJ, Lysyk TJ. Prevalence of Anaplasma bovis in Canadian populations of the Rocky Mountain wood tick, Dermacentor andersoni. Ticks Tick Borne Dis. 2018;9:1528–31. DOIPubMedGoogle Scholar
- Lado P, Luan B, Allerdice MEJ, Paddock CD, Karpathy SE, Klompen H. Integrating population genetic structure, microbiome, and pathogens presence data in Dermacentor variabilis. PeerJ. 2020;8:
e9367 . DOIPubMedGoogle Scholar - Lane RS, Mun J, Peribáñez MA, Fedorova N. Differences in prevalence of Borrelia burgdorferi and Anaplasma spp. infection among host-seeking Dermacentor occidentalis, Ixodes pacificus, and Ornithodoros coriaceus ticks in northwestern California. Ticks Tick Borne Dis. 2010;1:159–67. DOIPubMedGoogle Scholar
- Zhuang L, Du J, Cui XM, Li H, Tang F, Zhang PH, et al. Identification of tick-borne pathogen diversity by metagenomic analysis in Haemaphysalis longicornis from Xinyang, China. Infect Dis Poverty. 2018;7:45. DOIPubMedGoogle Scholar
- Sumner JW, Nicholson WL, Massung RF. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–92. DOIPubMedGoogle Scholar
- Rar VA, Livanova NN, Panov VV, Doroschenko EK, Pukhovskaya NM, Vysochina NP, et al. Genetic diversity of Anaplasma and Ehrlichia in the Asian part of Russia. Ticks Tick Borne Dis. 2010;1:57–65. DOIPubMedGoogle Scholar
- Rar V, Livanova N, Tkachev S, Kaverina G, Tikunov A, Sabitova Y, et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit Vectors. 2017;10:258. DOIPubMedGoogle Scholar
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. DOIPubMedGoogle Scholar
- Sashika M, Abe G, Matsumoto K, Inokuma H. Molecular survey of Anaplasma and Ehrlichia infections of feral raccoons (Procyon lotor) in Hokkaido, Japan. Vector Borne Zoonotic Dis. 2011;11:349–54. DOIPubMedGoogle Scholar
- Goethert HK, Telford SR III. Enzootic transmission of Anaplasma bovis in Nantucket cottontail rabbits. J Clin Microbiol. 2003;41:3744–7. DOIPubMedGoogle Scholar
- Yabsley MJ, Romines J, Nettles VF. Detection of Babesia and Anaplasma species in rabbits from Texas and Georgia, USA. Vector Borne Zoonotic Dis. 2006;6:7–13. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: August 16, 2023
1Current affiliation: Agriculture and Agri-food Canada, Lethbridge, Alberta, Canada.
2Current affiliation: Mayo Clinic, Jacksonville, Florida, USA.
Table of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sandor E. Karpathy, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H17-3, Atlanta, GA 30329-4027, USA
Top