Volume 30, Number 2—February 2024
Research
Impact of Meningococcal ACWY Vaccination Program during 2017–18 Epidemic, Western Australia, Australia
Figure 1
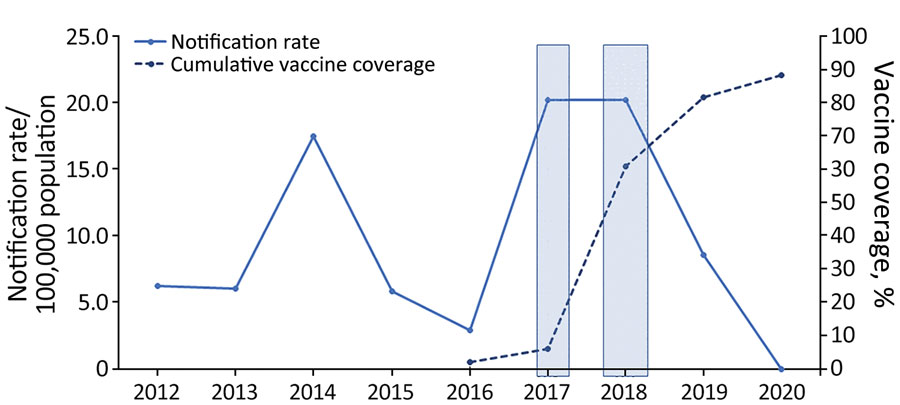
Figure 1. Invasive meningococcal disease notification rates (solid line) and cumulative meningococcal ACWY vaccine coverage rates (dotted line) among children 12 months–4 years of age, Western Australia, 2012–2020. First shaded box indicates the period of December 2016–March 2017, during which the local Western Australia government funded the meningococcal ACWY conjugate vaccine for children 12 months–4 years of age and adolescents 15–19 years of age in selected affected and at-risk regional areas. Second shaded box indicates period of October–December 2017, during which vaccine was funded for people of all ages in selected affected and at-risk regional areas. Beginning in January 2018, the vaccine was funded and available to all children 12 months–4 years of age.
1These authors contributed equally to this article.