Volume 30, Number 2—February 2024
Dispatch
Emerging Enterovirus A71 Subgenogroup B5 Causing Severe Hand, Foot, and Mouth Disease, Vietnam, 2023
Abstract
We report on a 2023 outbreak of severe hand, foot, and mouth disease in southern Vietnam caused by an emerging lineage of enterovirus A71 subgenogroup B5. Affected children were significantly older than those reported during previous outbreaks. The virus should be closely monitored to assess its potential for global dispersal.
Since 1997, large outbreaks of severe hand, foot, and mouth disease (HFMD) caused by diverse enterovirus A71 (EV-A71) subgenogroups (such as B4, B5, C4, and C5) have been reported in the Asia Pacific region (1), resulting in millions of hospitalizations and substantial numbers of deaths. Increased EV-A71 detection and associated neurologic disease have also been documented worldwide, including in the United States in more recent years (2).
During January 1–June 30, 2023, a total of 12,600 HFMD cases and 7 deaths were reported in Vietnam. Of those cases, 5,383 (42.7%) infections and all 7 deaths were recorded in June 2023. We investigated the epidemiologic and virologic features of this outbreak. The study was approved by the Institutional Review Board of CH1 and the Oxford University Tropical Research Ethics Committee. Written informed consent was obtained from a parent or guardian of each enrolled patient.
This study forms part of an ongoing HFMD research program conducted at Children’s Hospital 1 (CH1) in Ho Chi Minh City, Vietnam, since 2013 (Appendix) (3). Recruited patients had clinical data recorded and throat and rectal swab samples collected for virologic investigation of EV-A71 and other enterovirus infections (Appendix Figure 1) (4,5). We extracted complementary data from hospital records or from a clinical study conducted during 2013–2018 (3).
We generated EV-A71 whole-genome sequences directly from virus-positive rectal or throat swab samples that had sufficient viral loads (PCR cycle threshold values of <30) by using a metagenomics-based approach, as previously described (6). We performed recombination analysis by using the Chimera, GENECONV, Maxchi, Bootscan, and Siscan algorithms available in RDP4 software (7). To assess virus evolution, we constructed maximum-likelihood phylogenetic trees for enterovirus viral protein 1 (VP1) and whole-genome sequences by using IQ-TREE (8); we obtained representative global sequences from GenBank for comparisons (Appendix Tables 1, 2).
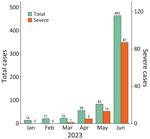
During January–June 2023, a total of 659 children with HFMD (including 106 with severe cases) were admitted to CH1; most admissions (463/659 [70.3%]) and severe cases (87/106 [82.1%]) occurred in June (Figure 1). Of the 659 children, 101 participated in this study. The participants resided in 15 provinces/cities in southern Vietnam (Appendix Figure 2) and were admitted to CH1 shortly after illness onset; the median number of illness days before admission was 2 (interquartile range 1–2) (Table 1). Twenty-eight (27.7%) participants had a disease severity grade of 2A, and 73 (72.3%) had grade 2B1 or worse (Table 1). Disease progressed from lower to higher severity grade in 63 (62.4%) of 101 children; clinical manifestations progressed within 1 day after admission in 47 (74.6%) children (Appendix Figure 3).
We detected enteroviruses in samples from 84 (83.2%) of 101 patients. Of those 84 patients, 83 (98.8%) were positive for EV-A71, and 1 patient was positive for coxsackievirus A5. We determined the subgenogroup for 67 samples and assigned 65 samples to subgenogroup B5 (Table 1) and 2 samples to subgenogroup C1. The 2 C1-infected patients had grade 2B1 and grade 3 disease severity. Compared with EV-A71–infected children enrolled in the clinical study during 2013–2018, those in the 2023 outbreak were significantly older (Table 2; Appendix Figure 4).
We obtained whole-genome sequences from 16 B5-positive samples (14 rectal and 2 throat swab samples from 16 individual patients) (Appendix Table 2). We did not detect recombination events. Phylogenetic analysis indicated the B5 viruses in Vietnam were most closely related to the B5 viruses from Japan, but they formed a distinct lineage from those previously isolated from Vietnam and worldwide (Figure 2; Appendix Table 3, Figure 5). In addition, 15 of 16 B5 sequences from the 2023 outbreak carried a glycine residue at position 17 (G17) within the N-terminus of VP1. In the 1 remaining sample, a G17 codon was detected in 3 of 122 reads generated by the metagenomic workflow, and a serine (S17) codon was detected in the remaining 119 reads (Appendix Figure 6). In contrast, among 287 nonidentical global B5 sequences used for phylogenetic analysis, an S17 codon was observed in 285 (99.3%) and a G17 codon was observed in 2 (0.7%) sequences. However, the 2 G17-containing sequences were derived from virus isolates passaged in cultured cell lines (9). Because of the small number of subgenogroup C1 sequences (n = 2), we deemed a similar in-depth analysis to be uninformative, but the C1 viruses from this study were closely related phylogenetically to C1 strains isolated worldwide (Appendix Figure 7).
We report that the 2023 outbreak of severe HFMD in Vietnam was caused by EV-A71 subgenogroups B5 and C1; B5 is dominant, and more older children were affected than during previous outbreaks. Phylogenetic analyses suggest that both B5 and C1 viruses were derived from new introductions of EV-A71 into Vietnam. In addition, the B5 viruses likely represent an emerging lineage because of a unique nonsynonymous amino acid substitution (S17G) in VP1 and because they form a distinct lineage within the global B5 phylogenetic tree. Further research is needed to clarify the origin and transmission network of this emerging lineage.
Underlying factors might cause the emergence of EV-A71 subgenogroups within a specific locality; the accumulation of a sufficient number of susceptible young children in the population and pathogen evolution might play critical roles (9,10). The changing epidemiology of respiratory pathogens as a consequence of COVID-19 has been documented (11), although EV-A71 is mainly transmitted by the oral-fecal route; thus, the effects of COVID-19 on EV-A71 transmission might be different from those of other respiratory viruses. However, the COVID-19 pandemic could have resulted in a large cohort of children who had greater susceptibility to EV-A71 infection, leading to a surge in infections among older children in the 2023 outbreak. Virus immune evasion or altered virulence might also be substantial contributing factors in the outbreak (9,12). The amino acid residue 17 in VP1 does not form part of the identified EV-A71 immune epitopes (13), but mutations in the N terminus of VP1 might increase cell tropism, potentially contributing to EV-A71 pathogenesis. Collectively, because VP1 is the most immunogenic protein of EV-A71, the potential effects of the nonsynonymous S17G substitution on immune escape and virulence of EV-A71 subgenogroup B5 warrant further investigation.
Previous peaks of EV-A71 outbreaks in Vietnam occurred during September–November (3), coinciding with school reopening after the summer holiday (June–August). As of November 2023, the outbreak in Vietnam was still ongoing and had resulted in >100,000 infections and 23 deaths across the country. The potential for severe EV-A71–associated HFMD outbreaks to spread to other parts of the world should be closely monitored.
Inactivated EV-A71 vaccines have been developed in China and Taiwan (14) but have only been used in China. Real-world data have shown that those vaccines substantially reduced EV-A71–associated disease transmission in China (15). Thus, using EV-A71 vaccines in other HFMD-endemic countries could have a similar effect. However, the extent to which EV-A71 vaccines might shape HFMD dynamics as a whole should be closely monitored. Because HFMD is transmitted through the oral-fecal route, good hygiene is critical to reduce EV-A71 transmission.
In conclusion, the 2023 outbreak of severe HFMD in Vietnam has mainly been caused by an emerging EV-A71 subgenogroup B5 lineage, and older children have been affected. Clinicians should recognize the diverse clinical manifestations of HFMD. Furthermore, enhanced EV-A71 surveillance is needed to inform the outbreak response in Vietnam and elsewhere, should the virus spread.
Dr. Chau is vice director of the Ho Chi Minh City Department of Health in Vietnam. His research interests focus on infectious diseases of public health importance in Vietnam, especially emerging infections.
This work was supported by the Wellcome Trust, United Kingdom (grant nos. 226120/Z/22/Z, 222574/Z/21/Z, and 225437/Z/22/Z). The funding body did not have any influence on the study design, study conduct, preparation of the manuscript, or decision to publish.
Acknowledgment
We thank the patients and their parents for their participation in this study and all nursing and medical staff in the pediatric intensive care unit and infectious diseases wards at Children’s Hospital 1 who provided care for the patients and helped collect clinical data.
References
- World Health Organization Regional Office for the Western Pacific. A guide to clinical management and public health response for hand, foot and mouth disease (HFMD). 2011 [cited 2023 Jun 1]. https://apps.who.int/iris/handle/10665/207490
- Messacar K, Burakoff A, Nix WA, Rogers S, Oberste MS, Gerber SI, et al. Notes from the field: enterovirus A71 neurologic disease in children—Colorado, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1017–8. DOIPubMedGoogle Scholar
- Nhan LNT, Khanh TH, Hong NTT, Van HMT, Nhu LNT, Ny NTH, et al. Clinical, etiological and epidemiological investigations of hand, foot and mouth disease in southern Vietnam during 2015 - 2018. PLoS Negl Trop Dis. 2020;14:
e0008544 . DOIPubMedGoogle Scholar - Thanh TT, Anh NT, Tham NT, Van HMT, Sabanathan S, Qui PT, et al. Validation and utilization of an internally controlled multiplex Real-time RT-PCR assay for simultaneous detection of enteroviruses and enterovirus A71 associated with hand foot and mouth disease. Virol J. 2015;12:85. DOIPubMedGoogle Scholar
- Kroneman A, Vennema H, Deforche K, v d Avoort H, Peñaranda S, Oberste MS, et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol. 2011;51:121–5. DOIPubMedGoogle Scholar
- Dung NT, Hung LM, Hoa HTT, Nga LH, Hong NTT, Thuong TC, et al. Monkeypox virus infection in 2 female travelers returning to Vietnam from Dubai, United Arab Emirates, 2022. Emerg Infect Dis. 2023;29:778–81. DOIPubMedGoogle Scholar
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:
vev003 . DOIPubMedGoogle Scholar - Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:1530–4. DOIPubMedGoogle Scholar
- Kobayashi K, Nishimura H, Mizuta K, Nishizawa T, Chu ST, Ichimura H, et al. Virulence of enterovirus A71 fluctuates depending on the phylogenetic clade formed in the epidemic year and epidemic region. J Virol. 2021;95:
e0151521 . DOIPubMedGoogle Scholar - Takahashi S, Metcalf CJE, Arima Y, Fujimoto T, Shimizu H, Rogier van Doorn H, et al. Epidemic dynamics, interactions and predictability of enteroviruses associated with hand, foot and mouth disease in Japan. J R Soc Interface. 2018;15:
20180507 . DOIPubMedGoogle Scholar - Eden JS, Sikazwe C, Xie R, Deng YM, Sullivan SG, Michie A, et al.; Australian RSV study group. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13:2884. DOIPubMedGoogle Scholar
- Tee KK, Lam TT, Chan YF, Bible JM, Kamarulzaman A, Tong CYM, et al. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol. 2010;84:3339–50. DOIPubMedGoogle Scholar
- Yuan J, Shen L, Wu J, Zou X, Gu J, Chen J, et al. Enterovirus A71 proteins: structure and function. Front Microbiol. 2018;9:286. DOIPubMedGoogle Scholar
- Nguyen TT, Chiu CH, Lin CY, Chiu NC, Chen PY, Le TTV, et al. Efficacy, safety, and immunogenicity of an inactivated, adjuvanted enterovirus 71 vaccine in infants and children: a multiregion, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2022;399:1708–17. DOIPubMedGoogle Scholar
- Hong J, Liu F, Qi H, Tu W, Ward MP, Ren M, et al. Changing epidemiology of hand, foot, and mouth disease in China, 2013-2019: a population-based study. Lancet Reg Health West Pac. 2022;20:
100370 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: January 18, 2024
1Members are listed at the end of this article.
Table of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Le Van Tan, Oxford University Clinical Research Unit, 764 Vo Van Kiet, District 5, Ho Chi Minh City, Vietnam
Top