Volume 30, Number 3—March 2024
Dispatch
Emergence of Thelaziosis Caused by Thelazia callipaeda in Dogs and Cats, United States
Abstract
We report 2 autochthonous feline thelaziosis cases caused by the eyeworm Thelazia callipaeda and discuss the spread among dogs in the northeastern United States. Phylogenetic analysis suggests the parasite was introduced from Europe. Adopting a One Health approach is needed to limit further spread of T. callipaeda eyeworms in North America.
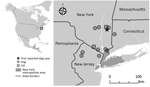
Thelazia callipaeda eyeworm was considered an exotic parasite in North America until an autochthonous case was reported in a dog from New York, USA, in 2020 (1). T. callipaeda eyeworm has been reported in countries in East Asia and the Soviet Union, later expanding its geographic range into Europe (2,3). This zoonotic parasite primarily infects the orbital cavity of its host causing thelaziosis (3). The zoophilic secretophagous male fly, Phortica variegata, is a T. callipaeda vector; flies ingest first-stage T. callipaeda larvae from the lacrimal secretions of an infected host and redeposit them as infective third-stage larvae, which eventually complete their life cycle by developing into adult worms (4). P. variegata flies have been found in Orange and Monroe Counties in New York (5,6), which has likely promoted the emergence of T. callipaeda eyeworm in North America (4). Since the T. callipaeda infection in a dog reported in New York in 2020, a total of 11 canine cases (6 in New York, 3 in New Jersey, 1 each in Connecticut and Nevada) and 2 feline cases (both from New York) (Figure 1) have been confirmed morphologically at the Cornell Animal Health Diagnostic Center (AHDC) in Ithaca, New York, USA. We describe 2 feline thelaziosis cases and discuss new canine cases in northeastern United States (New York/New Jersey border) during February 2021–December 2022 and One Health approaches to limit spread of this emerging disease in the United States.
Case 1 was in a 16-year-old neutered male, domestic shorthair cat from Greenwood Lake, Orange County, New York, that had been regularly cared for at the Warwick Valley Veterinary Hospital in New York, since October 2019. The animal had a recurrent history of flea infestation, which was managed with selamectin. The cat received routine rabies vaccinations at the clinic and was regularly dewormed with a combination of emodepside (3 mg/kg) and praziquantel (12 mg/kg) applied topically to the skin by the owner. Since June 2021, the animal has been treated for progressive chronic kidney disease. During a visit in April 2022, the cat had crusty lesions on its swollen right eye. Initial treatment with an ophthalmic ointment containing tobramycin resolved the eye infection. In August 2022, the cat manifested squinting, epiphora, and mucus accumulation in the right eye, which did not improve after tobramycin treatment. Detailed examination of the right eye revealed a constricted pupil and an elevated nictitating membrane with 4 thread-like worms, which were recovered mechanically at the clinic by flushing with saline solution. Of the 4 worms collected, 1 intact worm was received at AHDC for identification. The cat did not travel outside of New York. The animal was prescribed an ophthalmic ointment containing neomycin and polymyxin B and a dewormer (combination of emodepside [3 mg/kg] and praziquantel [12 mg/kg]) applied topically to the skin. No relapse was observed after treatment.
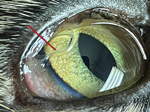
Case 2 was in a 2.5-year-old spayed female, domestic shorthair cat from a multicat household in Clinton Corners, Dutchess County, New York (adopted in Columbia County, New York). The cat did not travel outside of New York and was examined in October 2022 at a pet hospital during a routine rabies vaccination appointment. Ophthalmic examination revealed multiple white thread-like worms on the bulbar conjunctiva of both eyes (Figure 2). The cat had no clinical signs and was prescribed a dewormer (combination of emodepside [3 mg/kg] and praziquantel [12 mg/kg]) applied topically to the skin. Follow-up after 2 weeks revealed the presence of 8 worms, which were manually removed under local anesthesia. Two intact worms were sent to AHDC for identification. The cat was prescribed a combination of imidacloprid (10 mg/kg) and moxidectin (1 mg/kg) applied topically to the skin. Complete recovery was noted during a follow-up visit in November 2022.
At AHDC, we identified 1 male worm from case 1 and 2 female worms from case 2 morphologically as T. callipaeda eyeworm, primarily on the basis of transverse cuticular striations. The female worms were 11 and 14 mm long, and the male worm was 8.1 mm long; all 3 had a wide, moderately deep buccal cavity. The number of transverse cuticular striations at the cephalic, midbody, and caudal regions ranged 150–400/mm/region in both male and female worms. In the male worm, the long spicule was ≈2 mm long and the short spicule was 0.1 mm long. The vulval opening in the female worms was anterior to the esophageal/intestinal junction (Appendix Figure 1).
We performed PCR on 1 female worm sample from feline case 2 and 1 sample from a dog case targeting 12S rRNA, 18S rRNA, and cytochrome oxidase c subunit 1 (cox1) using previously described protocols (7–9). The amplified PCR products for both worm samples were 421 bp for 12S rRNA, 891 bp for 18S rRNA, and 612 bp for cox1. We Sanger sequenced the PCR products, edited and aligned the sequences by using BioEdit (https://bioedit.software.informer.com), and compared them with available GenBank sequences by using BLAST analysis (https://blast.ncbi.nlm.nih.gov). We observed 100% sequence identity with corresponding genes available for T. callipaeda in GenBank. We deposited the sequences from this study in GenBank under accession nos. OR545549, OR545261, and OR982681. Phylogenetic analysis of the cox1 sequences revealed clustering as a monophyletic clade with T. callipaeda haplotype 1 from Europe (10,11) (Appendix Figure 2). This study and the previous report on a dog (1) reconfirm the possibility that this parasite was introduced from Europe and subsequently spread in the United States.
The presence of T. callipaeda eyeworm in 2 cats and 11 dogs with no travel history outside of the United States suggests that this parasite is emerging in North America. Indeed, a previous study documented the presence of P. variegata flies in 2 counties in New York and indicated this fly species is a competent vector for T. callipaeda eyeworm, further suggesting an emerging threat by this eyeworm in the northeastern region of the United States (6). In addition, a wide variety of wildlife in New York, including coyotes, red foxes, gray foxes, black bears, raccoons, minks, least weasels, striped skunks, cottontail rabbits, and snowshoe hares, might act as potential hosts for T. callipaeda eyeworm (6); no human cases have been reported from this geographic area. A canine thelaziosis case was also found in the western United States (Nevada), although the travel history is unknown for that dog. Adopting proper diagnosis and surveillance measures is critical to limit the spread of this zoonotic parasite. Studies on control and treatment approaches for dogs suggest mechanical removal of adult and larval T. callipaeda nematodes coupled with the administration of diverse deworming drugs is effective (12). Because vector control using fly repellents is ineffective (3), control of T. callipaeda infections mainly rely on diagnosis and timely anthelmintic treatment. The presence of the natural vector, P. variegata flies (4,6), and the potential involvement of the sylvatic cycle promote the spread of this exotic parasite. Most cases in this study were diagnosed in late summer and autumn, which correlates with peak fly activity. Therefore, prophylactic anthelmintic administration coinciding with fly seasons would be an effective control strategy. Furthermore, as indicated in previous reports (1,4), adoption of a holistic One Health approach will be effective in further limiting the spread of T. callipaeda eyeworm in North America.
Dr. Manoj is a postdoctoral associate and Merck parasitology resident at the National Center for Veterinary Parasitology at the Animal Health Diagnostic Center, Cornell University, New York. Her research interests include vectorborne zoonotic diseases, particularly filarioids and their endosymbiont Wolbachia.
Acknowledgment
We thank Nicholas A. Hollingshead for his assistance in preparing the map and Antech Diagnostics for sharing information about Thelazia infection cases.
References
- Schwartz AB, Lejeune M, Verocai GG, Young R, Schwartz PH. Autochthonous Thelazia callipaeda infection in dog, New York, USA, 2020. Emerg Infect Dis. 2021;27:1923–6. DOIPubMedGoogle Scholar
- do Vale B, Lopes AP, da Conceição Fontes M, Silvestre M, Cardoso L, Coelho AC. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001-2020. Parasite. 2020;27:52. DOIPubMedGoogle Scholar
- Otranto D, Mendoza-Roldan JA, Dantas-Torres F. Thelazia callipaeda. Trends Parasitol. 2021;37:263–4. DOIPubMedGoogle Scholar
- Otranto D, Cantacessi C, Testini G, Lia RP. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int J Parasitol. 2006;36:1167–73. DOIPubMedGoogle Scholar
- Werner T, Steenwinkel T, Jaenike J. The encyclopedia of North American drosophilids: drosophilids of the midwest and northeast. 2018 [cited 2021 May 10]. https://digitalcommons.mtu.edu/cgi/viewcontent.cgi?article=1000&context=oabooks
- Otranto D, Iatta R, Lia RP, Cavalera MA, Màca J, Pombi M, et al. Competence of Phortica variegata from the United States as an intermediate host of the Thelazia callipaeda eyeworm. Am J Trop Med Hyg. 2018;98:1175–8. DOIPubMedGoogle Scholar
- Floyd RM, Rogers AD, Lambshead PJD, Smith CR. Nematode‐specific PCR primers for the 18S small subunit rRNA gene. Mol Ecol Notes. 2005;5:611–2. DOIGoogle Scholar
- Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. DOIPubMedGoogle Scholar
- Morales-Hojas R, Cheke RA, Post RJ. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J Helminthol. 2006;80:281–90.PubMedGoogle Scholar
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–7. DOIPubMedGoogle Scholar
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–74. DOIPubMedGoogle Scholar
- Bezerra-Santos MA, Mendoza-Roldan JA, Sgroi G, Lia RP, Venegoni G, Solari Basano F, et al. Efficacy of a formulation of sarolaner/moxidectin/pyrantel (Simparica Trio®) for the prevention of Thelazia callipaeda canine eyeworm infection. Parasit Vectors. 2022;15:370. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: February 13, 2024
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Manigandan Lejeune, Department of Population Medicine and Diagnostic Sciences, Cornell University College of Veterinary Medicine, Ithaca, NY 14853, USA
Top