Volume 30, Number 3—March 2024
Dispatch
Biphasic MERS-CoV Incidence in Nomadic Dromedaries with Putative Transmission to Humans, Kenya, 2022–2023
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) is endemic in dromedaries in Africa, but camel-to-human transmission is limited. Sustained 12-month sampling of dromedaries in a Kenya abattoir hub showed biphasic MERS-CoV incidence; peak detections occurred in October 2022 and February 2023. Dromedary-exposed abattoir workers (7/48) had serologic signs of previous MERS-CoV exposure.
Middle East respiratory syndrome coronavirus (MERS-CoV) is endemic in dromedary camels from the Arabian Peninsula and Africa; seroprevalence is >75% (1–3). Zoonotic transmission to humans has occurred sporadically, mainly on the Arabian Peninsula; >2,400 MERS cases and >800 deaths have occurred (4). Despite Kenya being a major camel-breeding country, only 3 potentially autochthonous camel-exposed humans with subclinical MERS-CoV infections were identified in 2019 (5). The apparent regional epidemiologic differences might be linked to factors such as limited diagnostics, local risk factors (e.g., human comorbidities, camel herding practices, seasonality), or MERS-CoV strain–specific features (6).
In farmed dromedary camels, MERS-CoV outbreaks were associated with annually synchronized camel parturition (7). In particular, camel calves tested MERS-CoV RNA–positive upon the loss of maternal antibodies 4–6 months after birth. Because of seasonality and changing food availability, most camels in Africa are nomadic and have variable population density. High population density is correlated with MERS-CoV seropositivity in camels in Kenya (1), but detailed insights into MERS-CoV circulation are missing.
Field studies on nomadic camels are hampered by limited infrastructure in remote and resource-restricted regions (8). However, nomadic camels are regularly transported to abattoir hubs, enabling sustained daily testing. We performed a continuous 12-month study at an abattoir hub in northern Kenya to investigate MERS-CoV incidence in nomadic camels and explore potential transmission to slaughterhouse workers.
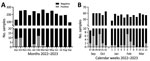
Our sampling site was an abattoir hub in Isiolo, northern Kenya, where camels from Marsabit, Samburu, and Isiolo counties are slaughtered (Appendix Figure 1). During September 2022–September 2023, we took samples from 10–15 dromedary camels 4–5 days per week (Appendix). The camels (n = 2,711) were originally from 12 different administrative wards, mainly from Laisamis in Marsabit County (n = 1,841, 67.9%) and Burat in Isiolo County (n = 578, 21.3%) (Table; Appendix Figure 1). MERS-CoV RNA was detected in 36/2,711 (1.3%) (Table; Figure 1) camels using quantitative reverse transcription PCR, which amplifies the upstream of the envelope E gene, and confirmed by open reading frame (ORF) 1ab quantitative reverse transcription PCR or sequencing (Appendix). The cumulative RNA positivity rate was higher in September–October 2022 at 19/381 (5.0%) compared with 17/727 (2.3%) in January–March 2023 (Figure 1). Incidence was biphasic, showing detection peaks in the first weeks of October 2022 (7/60, 11.7%) and February 2023 (7/58, 12.1%) (Figure 1, panel B). For 9/36 MERS-CoV–positive samples, we obtained ORF1ab sequences and performed phylogenetic analysis. The 9 ORF1ab sequences were highly similar (>99.93% nucleotide identity) and had 99.75%–99.78% nucleotide identity with the closest MERS-CoV relative identified in Akaki, Ethiopia, in 2019 (9). Phylogenetic analysis showed that the 9 sequences clustered as a monophyletic group within clade C2.2, which encompasses East Africa strains initially detected in Kenya in 2018 (10) (Appendix Figure 2). Those sequences represent 3 putative MERS-CoV outbreaks occurring contemporarily in camels in Kenya (Appendix Table 1).
To test whether biphasic MERS-CoV RNA–positivity is accompanied by increased MERS-CoV IgG levels, we tested randomized camel serum samples (n = 369/2,711) by MERS-CoV S1 ELISA (Appendix). MERS-CoV IgG levels showed a median optical density ratio (ODR) of 2.14 (95% CI 0.59–3.48) and a seroprevalence of 80.76% (298/369) (Appendix Figure 3, panel A). Lowest IgG levels were identified in June (median ODR 1.28, 95% CI 0.20–3.31), whereas the highest levels were seen in March (median ODR 2.72, 95% CI 1.67–3.76). MERS-CoV IgG levels were negatively associated with RNA-positivity (Odds ratio [OR] 0.20, 95% CI 0.09–0.44; p<0.0001) (Appendix Figure 3, panel B). RNA-positivity was negatively associated with the season (dry vs. wet, OR 0.14, 95% CI 0.06–0.30; p<0.0001). Male camels were more likely to be RNA positive (OR 3.94, 95% CI 0.86–29.2; p = 0.11) and less likely to be seropositive (OR 0.27, 95% CI 0.08–0.77; p = 0.021) than were female camels. Older animals (>3 years of age) were more likely to be seropositive (86%) than were animals ≤3 years of age (72%), but this difference was not statistically significant.
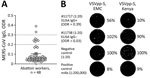
Seroepidemiologic studies have suggested that abattoir workers in contact with dromedaries are at increased risk for MERS-CoV exposure (11). Seroconversion of subclinical MERS cases might be missed when diagnostically implemented ELISA cutoffs of commercial kits (e.g., ODR = 1.1 for IgG positives) are applied (11,12). We identified MERS-CoV S1 IgG reactivity (ODR >0.2) in 7/48 (14.6%) of Isiolo abattoir workers (Figure 2, panel A). We excluded SARS-CoV-2 infection– or vaccine–induced antibody cross-reactivity with MERS-CoV S1 by comparison of ELISA ODRs of MERS-CoV S1–based with SARS-CoV-2 S1–based ELISA (Appendix Table 2, Figure 4). A control cohort (n = 12) with no history of camel exposure showed no MERS-CoV S1 IgG reactivity (0/12; 0%) despite high SARS-CoV-2 S1 IgG levels (11/12; 92%) (Appendix Table 2).
Neutralization tests (NT) based on GFP-encoding vesicular stomatitis virus pseudoparticles (VSVpp) carrying the MERS-CoV S protein from clade A EMC/2012 or clade C2.2 (Kenya) showed that 1/7 serum samples (1:20 dilution) had a VSVpp-NT 50% reduction of foci-forming units for EMC/2012 and a 90% reduction for Kenya VSVpp-S (Figure 2, panel B). A MERS-CoV EMC/2012-based plaque-reduction neutralization test (PRNT) showed a 50% PRNT at the 1:20 dilution, fulfilling the World Health Organization criteria for a confirmed MERS-CoV seroconversion. None of 6 selected MERS-CoV S1 ELISA-negative abattoir samples showed neutralizing capacity when tested by VSVpp-NT and PRNT (Appendix Table 2).
Our sustained sampling of dromedary camels showed a biphasic MERS-CoV incidence in northern Kenya not observed in previous studies (1,10,13). One explanation might be the short time of virus excretion in MERS-CoV–infected dromedaries (14), making viral RNA detection difficult without daily surveillance. Phylogenetic analysis suggests that we identified >3 MERS-CoV clusters over 3 different weeks in dromedaries originating from different wards. The first potential factor likely influencing the outbreaks is increased animal-to-animal interactions, because camels from different herds are transported to Isiolo and kept in holding pens together before slaughter, which could enhance MERS-CoV outbreaks. Second, increased interactions between immunologically naive and infected animals during transport and in holding pens increases the probability of transmitting MERS-CoV. That hypothesis is supported by the high percentage of IgG–negative adult camels (19.24%, ODR<0.3) (1,7). Although identifying the exact MERS-CoV transmission scenario between camels is logistically difficult, rapid point-of-care tests might help trace infections even in resource-limited conditions.
The overall biphasic MERS-CoV incidence might be linked to seasonal factors, such as the biannual alternating wet and dry seasons in northern Kenya. During dry seasons, herds congregate using limited forage, then migrate back to the point of origin in wet seasons. Because calves are mainly born during the 2 wet seasons, the loss of protection by maternal antibodies coincides with the dry seasons. Of note, the 2 dry seasons during July–October 2022 and January–February 2023 matched the peaks of MERS-CoV RNA–positivity in October 2022 and February 2023. The combination of immunologically naive, possibly infected camel calves and the dry season–specific increased population density and probability of contact at limited waterholes might encourage MERS-CoV infections and transmissions among camels.
We identified 7/48 abattoir workers with putative MERS-CoV exposure or past subclinical infection by implementing ELISA ODR cutoffs previously shown to be suitable for seroepidemiologic studies outside clinical settings. In 1/7 cases, we confirmed MERS-CoV neutralizing antibodies by VSVpp-based NT and PRNT. None of the abattoir workers experienced severe symptoms in recent years, supporting the hypothesis that clade C strains might have limited pathogenicity and transmissibility (15). Identifying defined factors that drive MERS-CoV outbreaks will assist in predictive epidemiology, risk assessment, and timely precautionary interventions for public and occupational health.
Mr. Ogoti is a virologist at the Center for Epidemiological Modelling Analysis (CEMA), University of Nairobi Institute of Tropical and Infectious Diseases, University of Nairobi, Kenya. His research interests include epidemiology and characterization of highly pathogenic coronaviruses.
Acknowledgments
We thank Patrick Muthui for excellent technical assistance, Muema Mulei for support in the initiation of the abattoir study, and Triza Shigoli, Noel Likalamu, and Andrea Sieberg for logistic and administrative assistance. We thank Gert Zimmer for the VSVpp system. We are grateful to all camel owners and abattoir workers for their help during the sample collection in Kenya. We thank the German FMD reference center at the Friedrich Löffler Institute, Insel Riems, Germany, for testing samples prior to import.
The work was funded by the German Research Foundation (DFG grant MU3564/3-1 to S.M.T and M.A.M.). C.D. received infrastructural support from the German Center for Infection Research (DZIF) and EU ERA-Net Project Durable (GA no. 101102733). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
M.A.M and V.M.C. are named on patents regarding SARS-CoV-2 serologic testing and monoclonal antibodies.
References
- Corman VM, Jores J, Meyer B, Younan M, Liljander A, Said MY, et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992-2013. Emerg Infect Dis. 2014;20:1319–22. DOIPubMedGoogle Scholar
- Meyer B, Müller MA, Corman VM, Reusken CB, Ritz D, Godeke GJ, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552–9. DOIPubMedGoogle Scholar
- Müller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, et al. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg Infect Dis. 2014;20:2093–5. DOIPubMedGoogle Scholar
- World Health Organization. Disease outbreak news: Middle East respiratory syndrome—United Arab Emirates [cited 2024 Feb 4]. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON478
- Munyua PM, Ngere I, Hunsperger E, Kochi A, Amoth P, Mwasi L, et al. Low-level Middle East respiratory syndrome coronavirus among camel handlers, Kenya, 2019. Emerg Infect Dis. 2021;27:1201–5. DOIPubMedGoogle Scholar
- Peiris M, Perlman S. Unresolved questions in the zoonotic transmission of MERS. Curr Opin Virol. 2022;52:258–64. DOIPubMedGoogle Scholar
- Meyer B, Juhasz J, Barua R, Das Gupta A, Hakimuddin F, Corman VM, et al. Time course of MERS-CoV infection and immunity in dromedary camels. Emerg Infect Dis. 2016;22:2171–3. DOIPubMedGoogle Scholar
- Gikonyo S, Kimani T, Matere J, Kimutai J, Kiambi SG, Bitek AO, et al. Mapping potential amplification and transmission hotspots for MERS-CoV, Kenya. EcoHealth. 2018;15:372–87. DOIPubMedGoogle Scholar
- Zhou Z, Ali A, Walelign E, Demissie GF, El Masry I, Abayneh T, et al. Genetic diversity and molecular epidemiology of Middle East Respiratory Syndrome Coronavirus in dromedaries in Ethiopia, 2017-2020. Emerg Microbes Infect. 2023;12:e2164218–27. DOIPubMedGoogle Scholar
- Kiambi S, Corman VM, Sitawa R, Githinji J, Ngoci J, Ozomata AS, et al. Detection of distinct MERS-Coronavirus strains in dromedary camels from Kenya, 2017. Emerg Microbes Infect. 2018;7:195. DOIPubMedGoogle Scholar
- Müller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–64. DOIPubMedGoogle Scholar
- Ko JH, Müller MA, Seok H, Park GE, Lee JY, Cho SY, et al. Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: performance analysis of serologic tests. Eur J Clin Microbiol Infect Dis. 2017;36:2179–86. DOIPubMedGoogle Scholar
- Ngere I, Hunsperger EA, Tong S, Oyugi J, Jaoko W, Harcourt JL, et al. Outbreak of Middle East respiratory syndrome coronavirus in camels and probable spillover infection to humans in Kenya. Viruses. 2022;14:1743–58. DOIPubMedGoogle Scholar
- Haagmans BL, van den Brand JM, Raj VS, Volz A, Wohlsein P, Smits SL, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. DOIPubMedGoogle Scholar
- Rodon J, Mykytyn AZ, Te N, Okba NMA, Lamers MM, Pailler-García L, et al. Extended viral shedding of MERS-CoV clade B virus in llamas compared with African clade C strain. Emerg Infect Dis. 2023;29:585–9. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: February 14, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcel A. Müller, Charité–Universitätsmedizin Berlin, Institute of Virology, Charitéplatz 1/Rahel-Hirsch-Weg 3, 10117 Berlin, Germany
Top