Volume 30, Number 4—April 2024
Research
Emergence of Poultry-Associated Human Salmonella enterica Serovar Abortusovis Infections, New South Wales, Australia
Abstract
Salmonella enterica serovar Abortusovis is a ovine-adapted pathogen that causes spontaneous abortion. Salmonella Abortusovis was reported in poultry in 2009 and has since been reported in human infections in New South Wales, Australia. Phylogenomic analysis revealed a clade of 51 closely related isolates from Australia originating in 2004. That clade was genetically distinct from ovine-associated isolates. The clade was widespread in New South Wales poultry production facilities but was only responsible for sporadic human infections. Some known virulence factors associated with human infections were only found in the poultry-associated clade, some of which were acquired through prophages and plasmids. Furthermore, the ovine-associated clade showed signs of genome decay, but the poultry-associated clade did not. Those genomic changes most likely led to differences in host range and disease type. Surveillance using the newly identified genetic markers will be vital for tracking Salmonella Abortusovis transmission in animals and to humans and preventing future outbreaks.
Salmonella enterica serovar Abortusovis is a host-restricted pathogen of sheep that causes invasive disease and spontaneous abortion (1–3). Transmission is thought to be limited to sheep; however, early studies identified other potential carriers (4). Because Salmonella Abortusovis is relatively rare, only a handful of studies have examined the molecular underpinning of its virulence. Other studies identified astA, sodC1, and sseI genes as virulence factors for invasive disease in lambs (5), and a mouse systemic infection model identified the spv toxin encoded on a plasmid as essential for virulence (6,7).
Previous examination of Salmonella Abortusovis epidemiology has exclusively relied on nongenomic molecular methods (8–11). Pulse-field gel electrophoresis (PFGE) and insertion sequence 200 fingerprinting have been used to identify clonal lineages in geographically distinct regions in Italy, Spain, Iran, and Switzerland (3,10–13). Cases and outbreaks in sheep have been described in several countries including Spain, Croatia, Switzerland, and Italy (8–10). However, the incidence of Salmonella Abortusovis animal infections in those and other countries is estimated to be underreported (9).
Salmonella Abortusovis is a reportable disease in sheep in Australia. Before 2009, the bacterium had not been reported in any animal (14). However, in 2009, Salmonella Abortusovis was detected in commercial poultry flocks in Australia (15) and was the second most common serovar in meat chickens in the country in 2016 (16). We examined the sudden emergence and proliferation of this serovar in poultry and humans in New South Wales (NSW), Australia, to investigate its evolution and implications to public health.
Isolate Sources and Metadata
We analyzed genomes and metadata of 56 Salmonella Abortusovis isolates (Table 1; Figure 1). Of those isolates, 51 were from Australia, 47 of which we sequenced in this study. Poultry isolates from Australia were collected during 2013–2019 and were divided into region A meat chickens (25 isolates), region B meat chickens (8 isolates), and egg-laying hens (6 isolates). Of the 33 isolates from regions A and B, 4 were from feed ingredients, 20 were from 10 farms in region A, and 8 were from 7 farms in region B. Multiple isolates were sampled from 5 region A farms and 1 region B farm. Human clinical isolates were collected during 2018–2020.
Sequencing and Assembly
We assembled draft genomes for all 47 isolates sequenced in this study and assembled complete genomes for 4 isolates (Table 1; Appendix 1; Appendix 2 Table 1). We submitted all raw sequencing data to the National Center for Biotechnology Information BioProject database (no. PRJNA993380).
Phylogenetic Analyses
We used the complete genome of strain 180121-R1 as the reference because it was the earliest representative of the poultry-associated clade isolate in Australia. We used Snippy version 4.6.0 (17) to call single-nucleotide polymorphisms (SNPs) for both assemblies and read sets and to generate core SNP alignments. We used the core SNP alignment as an input to custom Python scripts (https://github.com/mjohnpayne/Aus_Abortusovis) to perform pairwise distance analysis. Then we used IQ-TREE versions 2.0.3 (18) with default settings to generate a phylogeny for all 56 genomes. We generated a multilocus sequence typing (MLST)–based phylogeny from all sequence types (STs) within 5 alleles of ST768 and visualized all phylogenies by using iTOL (19) (Appendix 1).
Bayesian Analysis
We used BEAST version 2.6.3 (20) and 10,000,000 Markov chain Monte Carlo chain length to perform Bayesian phylogenetic reconstruction. A general time-reversible site model with a strict clock and coalescent constant population had the best effective sample size across 3 replicates, and we used that model to provide estimates of the most recent common ancestor (MRCA) date and evolutionary rate (Appendix 1).
Pan-Genome Analysis
We identified clade-specific genes by running roary version 3.13.0 (21) and default settings to define the pan-genome, and scoary version 1.6.16 (22) and default settings to identify clade-specific genes. We verified genes that were >80% sensitive and 100% specific for 1 clade by using KMA version 1.3.17 (23) and default settings to detect genes that were called absent because of assembly or annotation issues. We determined the presence of intact phages and plasmids in draft genomes by using KMA version 1.3.17 and default settings to search raw read data for phages and plasmids that were identified in complete assemblies.
Pseudogene and Genome Size Analysis
We used pseudofinder version 1.1 and default settings and a database of all uniprot protein sequences from Salmonella strains to detect pseudogenes (24). To compare genome size between the 2 Salmonella Abortusovis clades and exclude plasmids, we used the draft genome of isolate 405580-R1 to represent the poultry-associated clade because it lacks plasmids in the complete genome assembly. Similarly, the draft genome of ERR230420 appears to lack the pSLT-like plasmid likely found in the ovine-associated clade. We used draft genomes because the ovine-associated clade does not contain a complete genome for comparison and repetitive elements are often not assembled using Illumina (https://www.illumina.com) data. An isolate or isolate DNA was not readily available to generate a complete genome for the ovine-associated clade for this study.
Virulence Factor Identification
We searched all assemblies by using ABRicate version 1.0.1, the virulence factor database (VFDB), and plasmidfinder gene database by using mincov and minid set to 80 (25–27). We validated ABRicate results by using KMA version 1.3.17 for raw read mapping (Appendix 1). We used PHASTEST to identify prophages in complete genomes (28). We used the same datasets and approach used in a previous study to identify and select Salmonella Abortusovis–specific gene markers (29) (Appendix 1).
Distinct Poultry-Associated Clade
Phylogenetic analysis of the 56 isolates included in the study demonstrated that the 47 poultry and human clinical isolates from Australia represent a distinct poultry-associated clade that is at least 22,785 SNPs distant from the 9 Salmonella Abortusovis isolates from elsewhere, which formed an ovine-associated clade (Figure 2). The maximum pairwise SNP distance between isolates from Australia was only 42. Within the poultry-associated clade, we detected 2 subclades, 1 (subclade 1) containing 17 isolates and 1 (subclade 2) containing 34 isolates. Both subclades contained poultry isolates from regions A and B and human clinical isolates. Isolates from layer chickens were limited to a single lineage within subclade 2 and were not closely related to any human clinical isolates.
We also collected detailed source information that enabled a thorough examination of isolates at the farm level (Appendix 2 Table 2). We identified Salmonella Abortusovis in 2 feed ingredients, subclade 1 in canola and subclade 2 in blood meal, and in breeder hens that were progenitors of broiler chickens, suggesting possible modes of transmission. Among 17 farms, 6 had >1 isolate collected, 5 region A farms and 1 region B farm. Of note, 1 farm, A-4, contained isolates from both subclades 1 and 2, suggesting multiple introduction events (Figure 2). By contrast, all other farms contained isolates from only 1 subclade, and some were closely related across multiple years (i.e., farm B-6) suggesting long-term colonization by a single strain.
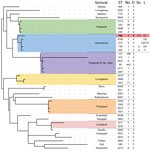
Because the poultry-associated clade was distant from other S. enterica Abortusovis strains, we examined the relationship of that clade to other serovars by using MLST. We located 5 additional Salmonella Abortusovis isolates in Enterobase (30). Those isolates had STs assigned but lacked genomic data, including 1 ovine isolate from Denmark (ST730) in 1920 and 1 from Italy (ST202) in 1980, plus 1 avian isolate from Australia in 2009 (ST786). We found that isolates from outside Australia that had genomic data were assigned to ST373 (6 isolates) and ST8760 (1 isolate). The ST assigned to the 2009 isolate from Australia, ST768, was also assigned to all isolates in the poultry-associated clade in this study. We identified all STs that were similar to ST768 and generated a phylogeny from MLST allele sequences. That phylogeny demonstrated that the poultry-associated clade was more closely related to the ovine-associated Salmonella Abortusovis clade than to any other serovars (Figure 3).
Estimation of MRCA for the Poultry-Associated Clade
Salmonella Abortusovis was observed in poultry in Australia beginning in 2009. We estimated the age of the poultry-associated clade by using temporal Bayesian analysis to determine whether that was the origin of the clade, or the clade was older. We estimated the date of the MRCA of the poultry-associated clade to be September 2004 (highest posterior density October 1998–August 2009) with a mutation rate of 2.43 × 10−7 (highest posterior density 1.55 to 3.28 × 10−7) SNPs/site/year, equivalent to 1.19 (range 0.76–1.61) SNPs/genome/year.
Plasmids in the Poultry-Associated Clade
To characterize the plasmid complement of the poultry-associated clade, we generated complete genomes, including closed plasmid sequences for 4 isolates: 180121-R1, 401964-R1, 405987-R1, and 405580-R1 (Table 2). We found that 405580-R1 carried no plasmids, but 180121-R1 and 401964-R1 contained the same 139-kb plasmid (pSAbAus), which carried the Yersinia high pathogenicity island (HPI) encoding yersiniabactin, a vertebrate lysozyme inhibitor (ivy), and a colicin secretion protein (cvaA). Isolate 405987-R1 carried a 60-kb plasmid that is identical to pSAbAus with a 79-kb deletion, which included the HPI, ivy, and cvaA loci. Isolate 401964-R1 also carried an additional 33-kb plasmid that is identical to pRHB32-C15_3 (GenBank accession no. CP057236.1) apart from 12 SNPs. Plasmid pRHB32-C15_3 was isolated from an Escherichia coli isolate on a sheep farm in the United Kingdom in 2017. We identified plasmid Inc types in all genomes and identified IncFIB(K)_1_Kpn3 in 45 genomes and on the 139-kb and 60-kb plasmids in complete genomes.
We examined draft genomes of the remaining isolates for plasmids that we had identified in the complete genomes, and only detected pSAbAus in the poultry-associated clade. We found the complete plasmid in 42 isolates, including 2 complete genomes, and it was partially extant in 2 isolates, including 405987-R1, but was absent from 7 isolates, including 405580-R1 (Figure 2). We detected the pRHB32-C15_3 plasmid in 13 poultry-associated clade isolates (Appendix 2 Table 3).
Three genomes in the ovine-associated clade contained an IncFII(S)_1 gene and Spv toxin encoding genes spvC, spvD, and spvR, which are typical of Salmonella virulence plasmids, such as pSLT from Salmonella Typhimurium. Those 3 strains also contained regions 35,184–38,729 bp in length that match to the pSLT plasmid at >97% identity. Taken together, those findings show that the 3 strains likely contain a Salmonella virulence plasmid. However, determining the makeup of that plasmid is difficult because no complete genomes were available in the ovine-associated clade. We did not observe any evidence of a classical virulence plasmid in the other 2 strains in the ovine-associated clade or the poultry-associated clade.
Prophage Variation within the Poultry-Associated Clade
We identified prophage complements of poultry-associated clade isolates in the 4 complete genomes and identified 6 intact prophages and 1 questionable prophage (Table 3). We used the intact prophages to infer the prophage complement of isolates without complete genomes (Table 3; Appendix 2 Table 3). Of those isolates, Gifsy-2 was in all isolates from the poultry-associated clade and was the only prophage carrying a virulence factor, cytolethal distending toxin gene cdtB (Figure 2). All prophages were most similar to prophages in other Salmonella serovars, except Abortus_SfV, which was most similar to a prophage from Shigella flexneri (31).
Pan-Genome Differences between Ovine- and Poultry-Associated Clades
To identify factors that might contribute to the different host range of the poultry- and ovine-associated clades, we examined pan-genomes to identify genes associated with either clade (Figure 2). There were 433 genes unique to the poultry-associated clade, 278 of which have no known function. Among the other 155 genes, 23 are found within prophages and 55 within the pSAbAus plasmid. The 77 chromosomal genes unique to the poultry-associated clade included 2 predicted fimbrial subunit genes, cfaB and stfF; an acid resistance chaperone protein gene, hdeB; and 10 (eutEJGHABCLKR) of the 17 (eutSPQTDMNEJGHABCLKR) genes of the ethanolamine utilization operon (Figure 2).
The ovine-associated clade contained 130 unique genes, 62 of which have no known function. Unique genes included astA and sodC1, which have previously been identified on a Gifsy-2 phage in this clade (5), as well as an outer membrane porin gene, ompN, and hcp1, a type 6 secretion system gene. However, because of the lack of a complete genome in the ovine-associated clade, we could not confirm the location of those specific genes.
All isolates contained intact or nearly intact chromosomal gene sets for Salmonella pathogenicity islands 1, 2, 3, and 24 (also called CS54) and for the enterobactin gene cluster and 3 types of fimbriae. We also detected virulence genes ompA and mig-14 in all isolates.
Ovine-Associated Clade Genomic Markers of Host Adaptation
Genome size and pseudogene number can be indicators of host restriction. Therefore, we compared those metrics between the ovine- and poultry-associated clades. A representative ovine-associated clade genome was 315 Kbp (7.1%) smaller than a representative poultry-associated clade genome (4.43 Mbp vs. 4.74 Mbp). The average ovine-associated clade genome contained 446 (range 384–667) pseudogenes, but the poultry-associated clade had 237 (range 224–248) pseudogenes (Appendix 1). That contrasting difference in the number of pseudogenes is similar in magnitude to generalist and host restricted Salmonella serovars. Generalist serovar Typhimurium has 201 and serovar Entertiditis has 320 pseudogenes; host-restricted serovar Typhi has 485 and serovar Gallinarium has 510 pseudogenes.
Identification and Validation of Salmonella Abortusovis Serovar-Specific Gene Markers
We searched for candidate-specific gene markers for the poultry-associated clade and for Salmonella Abortusovis in the accessory genomes of a dataset of 106 common serovars including Salmonella Abortusovis (Appendix 1). We identified 2 Salmonella Abortusovis markers and 2 poultry-associated clade markers with 100% sensitivity and 100% specificity (Table 4) and provided DNA sequences of those 4 Salmonella Abortusovis serovar-specific gene markers (Appendix 2 Table 1).
Salmonella Abortusovis is an ovine-adapted serovar and not known to cause disease in humans (3,6). The bacterium is endemic in several countries in Europe and Asia but has not been observed in the sheep population of Australia despite strong biosecurity surveillance and the serovar being notifiable (8–10,33). However, Salmonella Abortusovis was the second most frequent serovar in meat chickens in Australia in 2009 (14). We sequenced 47 isolates from poultry and human infections in Australia and compared those with 9 publicly available Salmonella Abortusovis genomes to understand the relationship between isolates from Australia and those from other countries and sources.
All isolates from Australia formed 1 closely related clade that is distant from the ovine-associated clade of Salmonella Abortusovis (Figures 1, 2). The 2004 MRCA date of the poultry-associated clade also indicates that it has existed in the NSW poultry flock for 15–20 years, a finding supported by the reported observation of Salmonella Abortusovis in NSW in 2009 (15). The relationships of the isolates sampled suggest that the serovar in poultry in Australia originated from a single introduction, however because no related isolates have been identified, the source is unknown.
Analysis of the pan genomes of all Salmonella Abortusovis isolates revealed substantial differences between the poultry-associated (433 unique genes) and ovine-associated clades (130 unique genes). A subset of those unique genes can be attributed to the 5 prophages and 2 plasmids only found in the poultry-associated clade and 1 putative plasmid only found in the ovine-associated clade.
Although all Salmonella Abortusovis isolates shared a complement of virulence factors, we noted some key differences. The poultry-associated clade carried Yersinia HPI, cvaA, and ivy on a plasmid, and cdtB on a prophage. The Yersinia HPI is a virulence factor in multiple human pathogens (34–37), and cdtB is a virulence factor in both typhoidal and nontyphoidal Salmonella (38). Other poultry-associated clade–specific genes included hdeB, which is known to contribute to acid resistance in Salmonella Enteritidis (39), and ivy, which encodes a vertebrate lysozyme inhibitor that improves survival in human saliva (40). Both genes could improve the chances of survival of the bacterium through the human upper gastrointestinal tract. The colicin export protein gene, cvaA, and the ethanolamine utilization operon, eut, found in 10 of 17 genes of the poultry-associated clade might contribute to infection by enabling colonization through competition with gut microbiota (41). Of note, ethanolamine in the host also triggers the eutR gene to activate SPI2 expression and increases intramacrophage survival (42). The fimbrial gene cfaB is upregulated in Salmonella Typhi human infection and stfF is upregulated in Salmonella Typhimurium chicken infection, but their contributions to survival and virulence are unknown (43,44).
In the ovine-associated clade, the sodC1 gene is essential for systemic disease in lambs (5). The Spv toxin was essential in a murine infection model (6,7). The spv genes and associated pSLT-like putative plasmid were only found in 3 of 5 ovine-associated clade isolates, suggesting that virulence within that clade might vary. Indeed, the severity of Salmonella Abortusovis infections in sheep is known to vary greatly (45). Virulence genes that are specific to the ovine-associated clade include ompN, which is associated with survival in macrophages in Salmonella Typhi (46), and hcp, which is required for killing of commensal bacteria in Salmonella Typhimurium (47). The clade-specific genes and gene sets described here might explain the potential differences in host range and disease type between the ovine-associated and poultry-associated clades.
Salmonella Abortusovis was previously thought to be host restricted in sheep (45). Host restriction often leads to adaptations, including a reduction in genome size and an increase in pseudogenization (48). The average number of pseudogenes in an ovine-associated clade isolate is double the average of a poultry-associated clade isolate, and the average genome was 7.1% smaller. The pseudogene count of the poultry-associated clade was also similar to those of generalist serovars, such as Salmonella Typhimurium and Salmonella Enteritidis, but the ovine-associated clade was similar to the host restricted serovars Salmonella Typhi and Salmonella Gallinarium. Those results suggest that the ovine-associated clade might be host adapted and the poultry-associated clade likely has a broader host range. That hypothesis is supported by the data in this study, namely, the ability of the poultry associated clade to infect both chickens and humans.
One of the plasmids in the poultry-associated clade is very closely related to an E. coli plasmid found on a sheep farm in the United Kingdom (49), suggesting that the poultry-associated clade was linked with sheep. However, the link is more likely to be indirect through the E. coli host.
The epidemiology of the isolates in this study demonstrated that the poultry-associated clade was widely distributed in NSW poultry and caused sporadic human infections. Salmonella Abortusovis was sampled across 8 years and was found in feed ingredients, in egg-laying hens, and in 20 different meat chicken farms from 2 regions. In addition, isolates from both region A and region B were found in each of the subclades in the phylogeny, suggesting that Salmonella Abortusovis has no geographic barriers. One exception was the egg layer hen population that formed a single group within subclade 2, indicating a single introduction. We also noted evidence for both long-term carriage of a single clone in 1 farm and multiple separate introductions into another farm. Isolation of Salmonella Abortusovis from feed ingredients suggests that transmission might have occurred through feed, but we found no identical isolates within short timespans in feed or chickens that would indicate direct transmission. Isolates from human infections were distributed across the phylogeny, suggesting that multiple separate transmission events to humans occurred; however, none caused large outbreaks. Salmonella Abortusovis does not efficiently transmit to humans from poultry (50), which might explain the low number of sporadic human cases despite the widespread detection in poultry.
The ability of the poultry-associated clade to colonize poultry and cause human disease, and the possibility that it might not cause systemic disease in lambs, make detection and differentiation of this clade from the ovine-associated clade useful. The genetic markers identified here will enable simple differentiation of the 2 Salmonella Abortusovis clades by using genomic data and potentially decrease root cause analysis time for detection of this novel Salmonella in the food industry. All markers described here could be used to produce PCR-based assays that would enable simple detection and differentiation of Salmonella Abortusovis clades, which is necessary because of their ability to either cause human infections or cause major losses of sheep flocks.
In conclusion, we examined the genomic epidemiology of Salmonella Abortusovis in poultry and human infections in NSW, Australia. The poultry-associated clade was only distantly related to existing examples of Salmonella Abortusovis and had key differences in virulence factors that suggest it might have differences in host range and disease type. Evidence suggests that the serotype has become endemic within the NSW poultry industry, where it can move between poultry facilities and to humans. Surveillance using the newly identified genetic markers will be vital for tracking transmission within poultry producing regions and to prevent any future outbreaks that could be caused by this serovar.
Dr. Payne is a postdoctoral research associate at the University of New South Wales Sydney in Sydney, NSW, Australia. His research interests focus on the use of genomics to describe the population structures and evolution of bacterial pathogens.
Acknowledgments
This work was supported by The National Health and Medical Research Council of the Australian Government (grant no. 1146938).
S.W. and A.P. are employed by Birling Laboratories and provided poultry isolates and metadata.
References
- Jack E. Salmonella Abortus Ovis—an atypical Salmonella. Vet Rec. 1968;82:558.
- Pardon P, Sanchis R, Marly J, Lantier F, Pépin M, Popoff M. [Ovine salmonellosis caused by Salmonella abortus ovis] [in French]. Ann Rech Vet. 1988;19:221–35.PubMedGoogle Scholar
- Belloy L, Decrausaz L, Boujon P, Hächler H, Waldvogel AS. Diagnosis by culture and PCR of Salmonella abortusovis infection under clinical conditions in aborting sheep in Switzerland. Vet Microbiol. 2009;138:373–7. DOIPubMedGoogle Scholar
- Vodas K, Marinov MF, Shabanov M. [Salmonella abortus ovis carrier state in dogs and rats] [in Bulgarian]. Vet Med Nauki. 1985;22:10–5.PubMedGoogle Scholar
- Bacciu D, Falchi G, Spazziani A, Bossi L, Marogna G, Leori GS, et al. Transposition of the heat-stable toxin astA gene into a gifsy-2-related prophage of Salmonella enterica serovar Abortusovis. J Bacteriol. 2004;186:4568–74. DOIPubMedGoogle Scholar
- Colombo MM, Leori G, Rubino S, Barbato A, Cappuccinelli P. Phenotypic features and molecular characterization of plasmids in Salmonella abortusovis. Microbiology. 1992;138:725–31.
- Uzzau S, Gulig PA, Paglietti B, Leori G, Stocker BA, Rubino S. Role of the Salmonella abortusovis virulence plasmid in the infection of BALB/c mice. FEMS Microbiol Lett. 2000;188:15–8. DOIPubMedGoogle Scholar
- Habrun B, Listes E, Spicic S, Cvetnic Z, Lukacevic D, Jemersic L, et al. An outbreak of Salmonella Abortusovis abortions in sheep in south Croatia. J Vet Med B Infect Dis Vet Public Health. 2006;53:286–90. DOIPubMedGoogle Scholar
- Wirz-Dittus S, Belloy L, Hüssy D, Waldvogel AS, Doherr MG. Seroprevalence survey for Salmonella Abortusovis infection in Swiss sheep flocks. Prev Vet Med. 2010;97:126–30. DOIPubMedGoogle Scholar
- Valdezate S, Astorga R, Herrera-León S, Perea A, Usera MA, Huerta B, et al. Epidemiological tracing of Salmonella enterica serotype Abortusovis from Spanish ovine flocks by PFGE fingerprinting. Epidemiol Infect. 2007;135:695–702. DOIPubMedGoogle Scholar
- Dionisi AM, Carattoli A, Luzzi I, Magistrali C, Pezzotti G. Molecular genotyping of Salmonella enterica Abortusovis by pulsed field gel electrophoresis. Vet Microbiol. 2006;116:217–23. DOIPubMedGoogle Scholar
- Schiaffino A, Beuzón CR, Uzzau S, Leori G, Cappuccinelli P, Casadesús J, et al. Strain typing with IS200 fingerprints in Salmonella abortusovis. Appl Environ Microbiol. 1996;62:2375–80. DOIPubMedGoogle Scholar
- Nikbakht GH, Raffatellu M, Uzzau S, Tadjbakhsh H, Rubino S. IS200 fingerprinting of Salmonella enterica serotype Abortusovis strains isolated in Iran. Epidemiol Infect. 2002;128:333–6. DOIPubMedGoogle Scholar
- Australian Government Department of Agriculture, Fisheries and Forestry. Animal Health Australia 2009. Canberra (ACT), Australia: The Department; 2010.
- Heuzenroeder MW, Ross IL, Hocking H, Davos D, Young CC, Morgan G. An integrated typing service for the surveillance of Salmonella in chickens. Barton (ACT): Rural Industries Research and Development Corporation, Australian Government; 2013.
- Abraham S, O’Dea M, Sahibzada S, Hewson K, Pavic A, Veltman T, et al. Escherichia coli and Salmonella spp. isolated from Australian meat chickens remain susceptible to critically important antimicrobial agents. PLoS One. 2019;14:
e0224281 . DOIPubMedGoogle Scholar - Seemann T. snippy: fast bacterial variant calling from NGS reads, version 3.1 [cited 2020 Apr 19]. https://github.com/tseemann/snippy
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:1530–4. DOIPubMedGoogle Scholar
- Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44(W1):
W242-5 . DOIPubMedGoogle Scholar - Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2019;15:
e1006650 . DOIPubMedGoogle Scholar - Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3. DOIPubMedGoogle Scholar
- Brynildsrud O, Bohlin J, Scheffer L, Eldholm V. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016;17:238. DOIPubMedGoogle Scholar
- Clausen PTLC, Aarestrup FM, Lund O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics. 2018;19:307. DOIPubMedGoogle Scholar
- Syberg-Olsen MJ, Garber AI, Keeling PJ, McCutcheon JP, Husnik F. Pseudofinder: detection of pseudogenes in prokaryotic genomes. Mol Biol Evol. 2022;39:msac153.
- Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47(D1):D687–92. DOIPubMedGoogle Scholar
- Carattoli A, Hasman H. PlasmidFinder and in silico pMLST: identification and typing of plasmid replicons in whole-genome sequencing (WGS). Methods Mol Biol. 2020;2075:285–94. DOIPubMedGoogle Scholar
- Seemann T. ABRicate, mass screening of contigs for antimicrobial resistance or virulence genes. 1.0.1 [cited 2020 Apr 19]. https://github.com/tseemann/abricate
- Wishart DS, Han S, Saha S, Oler E, Peters H, Grant JR, et al. PHASTEST: faster than PHASTER, better than PHAST. Nucleic Acids Res. 2023;51(W1):W443–50. DOIPubMedGoogle Scholar
- Zhang X, Payne M, Lan R. In silico identification of serovar-specific genes for Salmonella serotyping. Front Microbiol. 2019;10:835. DOIPubMedGoogle Scholar
- Alikhan NF, Zhou Z, Sergeant MJ, Achtman M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018;14:
e1007261 . DOIPubMedGoogle Scholar - Allison GE, Angeles D, Tran-Dinh N, Verma NK. Complete genomic sequence of SfV, a serotype-converting temperate bacteriophage of Shigella flexneri. J Bacteriol. 2002;184:1974–87. DOIPubMedGoogle Scholar
- Zhang X, Payne M, Nguyen T, Kaur S, Lan R. Cluster-specific gene markers enhance Shigella and enteroinvasive Escherichia coli in silico serotyping. Microb Genom. 2021;7:
000704 . DOIPubMedGoogle Scholar - Clune T, Beetson S, Besier S, Knowles G, Paskin R, Rawlin G, et al. Ovine abortion and stillbirth investigations in Australia. Aust Vet J. 2020;1:22.PubMedGoogle Scholar
- Price SL, Vadyvaloo V, DeMarco JK, Brady A, Gray PA, Kehl-Fie TE, et al. Yersiniabactin contributes to overcoming zinc restriction during Yersinia pestis infection of mammalian and insect hosts. Proc Natl Acad Sci U S A. 2021;118:
e2104073118 . DOIPubMedGoogle Scholar - Wellawa DH, Allan B, White AP, Köster W. Iron-uptake systems of chicken-associated Salmonella serovars and their role in colonizing the avian host. Microorganisms. 2020;8:1203. DOIPubMedGoogle Scholar
- Schubert S, Picard B, Gouriou S, Heesemann J, Denamur E. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect Immun. 2002;70:5335–7. DOIPubMedGoogle Scholar
- Liu D, Yang Y, Gu J, Tuo H, Li P, Xie X, et al. The Yersinia high-pathogenicity island (HPI) carried by a new integrative and conjugative element (ICE) in a multidrug-resistant and hypervirulent Klebsiella pneumoniae strain SCsl1. Vet Microbiol. 2019;239:
108481 . DOIPubMedGoogle Scholar - Miller RA, Wiedmann M. The cytolethal distending toxin produced by nontyphoidal Salmonella serotypes Javiana, Montevideo, Oranienburg, and Mississippi induces DNA damage in a manner similar to that of serotype Typhi. MBio. 2016;7:e02109–16. DOIPubMedGoogle Scholar
- Joerger RD, Choi S. Contribution of the hdeB-like gene (SEN1493) to survival of Salmonella enterica enteritidis Nal(R) at pH 2. Foodborne Pathog Dis. 2015;12:353–9. DOIPubMedGoogle Scholar
- Deckers D, Vanlint D, Callewaert L, Aertsen A, Michiels CW. Role of the lysozyme inhibitor Ivy in growth or survival of Escherichia coli and Pseudomonas aeruginosa bacteria in hen egg white and in human saliva and breast milk. Appl Environ Microbiol. 2008;74:4434–9. DOIPubMedGoogle Scholar
- Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108:17480–5. DOIPubMedGoogle Scholar
- Anderson CJ, Clark DE, Adli M, Kendall MM. Ethanolamine signaling promotes Salmonella niche recognition and adaptation during infection. PLoS Pathog. 2015;11:
e1005278 . DOIPubMedGoogle Scholar - Harris JB, Baresch-Bernal A, Rollins SM, Alam A, LaRocque RC, Bikowski M, et al. Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi. Infect Immun. 2006;74:5161–8. DOIPubMedGoogle Scholar
- Harvey PC, Watson M, Hulme S, Jones MA, Lovell M, Berchieri A Jr, et al. Salmonella enterica serovar typhimurium colonizing the lumen of the chicken intestine grows slowly and upregulates a unique set of virulence and metabolism genes. Infect Immun. 2011;79:4105–21. DOIPubMedGoogle Scholar
- Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res (Faisalabad). 2011;42:34. DOIPubMedGoogle Scholar
- Sabbagh SC, Lepage C, McClelland M, Daigle F. Selection of Salmonella enterica serovar Typhi genes involved during interaction with human macrophages by screening of a transposon mutant library. PLoS One. 2012;7:
e36643 . DOIPubMedGoogle Scholar - Sana TG, Flaugnatti N, Lugo KA, Lam LH, Jacobson A, Baylot V, et al. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc Natl Acad Sci U S A. 2016;113:E5044–51. DOIPubMedGoogle Scholar
- Langridge GC, Fookes M, Connor TR, Feltwell T, Feasey N, Parsons BN, et al. Patterns of genome evolution that have accompanied host adaptation in Salmonella. Proc Natl Acad Sci U S A. 2015;112:863–8. DOIPubMedGoogle Scholar
- AbuOun M, Jones H, Stubberfield E, Gilson D, Shaw LP, Hubbard ATM, et al.; On Behalf Of The Rehab Consortium. A genomic epidemiological study shows that prevalence of antimicrobial resistance in Enterobacterales is associated with the livestock host, as well as antimicrobial usage. Microb Genom. 2021;7:10–2. DOIPubMedGoogle Scholar
- McLure A, Shadbolt C, Desmarchelier PM, Kirk MD, Glass K. Source attribution of salmonellosis by time and geography in New South Wales, Australia. BMC Infect Dis. 2022;22:14. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 11, 2024
Table of Contents – Volume 30, Number 4—April 2024
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Ruiting Lan, School of Biotechnology and Biomolecular Sciences, University of New South Wales Sydney, Sydney, NSW 2052, Australia
Top