Volume 9, Number 2—February 2003
Research
Annual Mycobacterium tuberculosis Infection Risk and Interpretation of Clustering Statistics
Figure 2
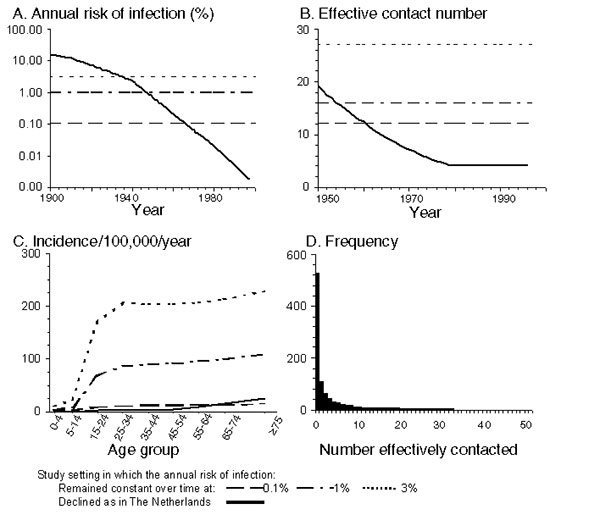
Figure 2. Summary of the assumptions defining contact between persons in the model. A, B, and C show the annual risk for infection, estimates of the average effective contact number in the model, and the average age-specific annual incidence of infectious disease per 100,000 population respectively in the various settings. For settings in which the annual risk for infection has not changed over time, the effective contact number is obtained from the ratio between the annual risk for infection and the incidence of infectious cases predicted in the model. The values for the effective contact number in the Netherlands are identical to those calculated in reference 6. D shows the frequency distribution of the assumed number of persons effectively contacted by each infectious case-patient, if the population were to comprise 1,000 infectious cases and the average effective contact number was approximately 4, as assumed for the Netherlands for recent years. This (negative binomial) distribution (defined by a variance 20 times the mean) led to observed cluster distributions that best compared against those observed in the Netherlands (6). Contact between persons is assumed to be assortative (so that, for example, those with a high-risk lifestyle, mix preferentially with similar persons) and, for simplicity, independent of age and sex.
References
- van Soolingen D, Borgdorff MW, de Haas PE, Sebek MM, Veen J, Dessens M, Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J Infect Dis. 1999;180:726–36. DOIPubMedGoogle Scholar
- Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–9. DOIPubMedGoogle Scholar
- Bauer J, Yang Z, Poulsen S, Andersen AB. Results from 5 years of nationwide DNA fingerprinting of Mycobacterium tuberculosis complex isolates in a country with a low incidence of M. tuberculosis infection. J Clin Microbiol. 1998;36:305–8.PubMedGoogle Scholar
- Styblo K, Meijer J, Sutherland I. The transmission of tubercle bacilli: its trend in a human population. Bull Int Union Tuberc. 1969;42:5–104.
- de Boer AS, Borgdorff MW, de Haas PEW, Nagelkerke NJ, van Embden JD, van Soolingen D. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J Infect Dis. 1999;180:1238–44. DOIPubMedGoogle Scholar
- Vynnycky E, Nagelkerke N, Borgdorff MW, van Soolingen D, van Embden JDA, Fine PEM. The effect of age and study duration on the relationship between ‘clustering’ of DNA fingerprint patterns and the proportion of tuberculosis disease attributable to recent transmission. Epidemiol Infect. 2001;126:43–62. DOIPubMedGoogle Scholar
- Holm J. Development from tuberculous infection to tuberculous disease. The Hague, the Netherlands: Royal Dutch Tuberculosis Assocation (KNCV); 1969.
- Sutherland I, Švandová E, Radhakrishna SE. The development of clinical tuberculosis following infection with tubercle bacilli. Tubercle. 1982;63:255–68. DOIPubMedGoogle Scholar
- Vynnycky E, Fine PEM. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. DOIPubMedGoogle Scholar
- Sutherland I, Bleiker MA, Meijer J, Styblo K. The risk of infection in the Netherlands from 1967 to 1979. Tubercle. 1983;64:241–53. DOIPubMedGoogle Scholar
- Fine PEM, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–75.PubMedGoogle Scholar
- van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. DOIPubMedGoogle Scholar
- Sutherland I. The ten-year incidence of clinical tuberculosis following “conversion” in 2,550 individuals aged 14 to 19 years. The Hague, the Netherlands: Royal Dutch Tuberculosis Association (KNCV); 1968.
- Glynn JR, Bauer J, de Boer AS, Borgdorff MW, Fine PEM, Godfrey-Faussett P, Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int J Tuberc Lung Dis. 1999;3:1055–60.PubMedGoogle Scholar
- Yeh RW, Ponce de Leon A, Agasino CB, Hahn JA, Daley CL, Hopewell PC, Stability of Mycobacterium tuberculosis DNA genotypes. J Infect Dis. 1998;177:1107–11. DOIPubMedGoogle Scholar
- Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, Transmission of tuberculosis in New York City: an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–6. DOIPubMedGoogle Scholar
- Glynn JR. Resurgence of tuberculosis and the impact of HIV infection. Br Med Bull. 1998;54:579–93.PubMedGoogle Scholar
- Glynn JR, Warndorff DK, Fine PEM, Msiska GK, Munthali MM, Ponnighaus JM. The impact of HIV on morbidity and mortality from tuberculosis in sub-Saharan Africa: a study in rural Malawi and review of the literature. Health Transit Rev. 1997; 75–87.
- ten Asbroek AH, Borgdorff MW, Nagelkerke NJ, Sebek MM, Deville W, van Embden JD, Estimation of serial interval and incubation period of tuberculosis using DNA fingerprinting. Int J Tuberc Lung Dis. 1999;3:414–20.PubMedGoogle Scholar