Volume 17, Number 1—January 2011
Research
Concurrent Conditions and Human Listeriosis, England, 1999–2009
Abstract
The epidemiology of listeriosis in England and Wales changed during 2001–2008; more patients >60 years of age had bacteremia than in previous years. To investigate these changes, we calculated risk for listeriosis by concurrent condition for non–pregnancy-associated listeriosis cases reported to the national surveillance system in England during 1999–2009. Conditions occurring with L. monocytogenes infection were coded according to the International Classification of Diseases, 10th Revision, and compared with appropriate hospital episode statistics inpatient denominator data to calculate incidence rates/million consultations. Malignancies (especially of the blood), kidney disease, liver disease, diabetes, alcoholism, and age >60 years were associated with an increased risk for listeriosis. Physicians should consider a diagnosis of listeriosis when treating patients who have concurrent conditions. Providing cancer patients, who accounted for one third of cases, with food safety information might help limit additional cases.
Listeriosis is a rare but serious foodborne disease caused by the bacterium Listeria monocytogenes. Three groups of persons are disproportionately affected: the elderly, the immunocompromised, and pregnant women and their unborn or newborn infants. The clinical signs of disease in these persons include septicemia, meningitis, and miscarriage. Pregnant women can transmit the infection to the fetus, for whom the result can be deadly. However, these women may not have clearly overt signs or symptoms of infection. Case-fatality rates range from 20% to 50% (1). The susceptibility of healthy persons to symptomatic listeriosis is substantially less than that of persons with underlying conditions.
Persons with cancer, diabetes, AIDS, and liver or kidney disease are often predisposed to severe infection and death after infection with L. monocytogenes. This predisposition is a consequence of suppressed T-cell–mediated immunity (2) caused by the condition or its treatment. Similarly, pregnant women, the elderly, and those receiving immunosuppressive therapy are also at risk because of impaired or modulated immune function.
The epidemiology of listeriosis in England and Wales has changed since 2001 (3). Incidence has increased (2.1 cases/million population during 1990–2000 vs. 3.6 cases/million population during 2001–2009), and more cases have been found in persons >60 years of age who had bacteremia (but not meningitis). Similar patterns have been reported in other countries in Europe (4–6). The reasons for these changes are not fully understood, but they do not seem to be caused by surveillance artifacts and are not associated with sex, season, geography, ethnic or socioeconomic differences, underlying conditions, or L. monocytogenes subtype (3). We have showed that the increase occurred in persons with cancer or other conditions whose treatment included acid-suppressing medication (7). In view of recent trends, we examined national surveillance data for England to quantify the role of concurrent conditions in persons with listeriosis and stratified these conditions to examine risks for persons >60 years of age.
The Health Protection Agency Centre for Infections has coordinated national surveillance of listeriosis in England and Wales since 1990. Cases are included in the system by voluntary referral of cultures to the national reference laboratory or by electronic reporting of confirmed cases from local laboratories. Clinical data, including details of patients’ concurrent conditions, are subsequently sought from the consultant clinical microbiologist involved in the care of the case-patient. Microbiologic data from local and reference laboratories and clinical and risk factor data are linked for each case, deduplicated as necessary, and stored in a bespoke Microsoft Access database (Microsoft, Redmond, WA, USA) Access database.
A case of listeriosis is defined as a person with clinically compatible illness and from whom L. monocytogenes was isolated from a normally sterile site. Cases are subsequently classified as either non–pregnancy-associated (persons >1 month of age) or pregnancy-associated (a maternal–fetal or maternal–neonatal pair; such pairs were considered a single case). In this study, we included non–pregnancy-associated cases reported from laboratories in England for which a clinical questionnaire was available and showed that at least 1 reported concurrent condition was present. We included cases reported during April 1, 1999–March 31, 2009 because denominator data were arranged by fiscal years. These cases included sporadic cases and cases that were identified as being part of common source foodborne outbreaks.
Authors (P.M. and I.A.G.) reviewed each reported concurrent condition and assigned an International Classification of Diseases, 10th Revision (ICD-10) (8), code when appropriate. Rules for assigning codes were developed at the outset to ensure standardized coding throughout the study (Technical Appendix). These rules were validated by a third author (S.J.O.), a clinically qualified investigator, who also reviewed any coding disparities. Counts were calculated of all persons and those >60 years of age for each ICD-10 chapter (ICD-10 codes are aggregated into 22 chapters) and subgroup (within each chapter).
Hospital episode statistics finished consultant episodes (FCE) data, which were aggregated by ICD-10 code, age group (0–14 years, 15–59 years, 60–74 years, and >75 years), and fiscal year, were obtained from the Health and Social Care Information Centre (9) and used as denominator data. These data describe episodes of continuous admitted patient care under a specific consultant for National Health Service hospital inpatients in England, and a primary diagnosis is assigned to each episode by using ICD-10 coding. To ensure reliable confidence intervals (CIs), we calculated incidence rates/million FCEs and 95% CIs for each ICD-10 chapter and subgroup in which there were >10 cases. Two ICD-10 chapters not used by hospital episodes statistics to code primary diagnoses, external causes of morbidity and mortality (V01–Y98) and codes for special purposes (U00–U99), were not considered. Relative risks (RRs) and corresponding 95% CIs were calculated as appropriate when >10 cases were reported for a concurrent condition subgroup or chapter. Analysis was then repeated for case-patients >60 years of age.
Data were stored, manipulated, and summarized by using Microsoft Access, and incidence rates and RRs were calculated by using Microsoft Excel. Differences in proportions and changes in proportions over strata were assessed by using the χ2 test and the χ2 test for trend, respectively.
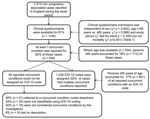
A total of 1,239 ICD-10–coded concurrent conditions were reported by 1,413 case-patients with non–pregnancy-associated listeriosis in England during April 1, 1999–March 31, 2009 (Figure). Of those patients who reported >1 underlying condition, 21 (2.2%) were identified as being part of a common source outbreak. Characteristics of case-patients with and without a completed clinical questionnaire are shown in Table 1. Overall, 9.1 cases of listeriosis/million FCEs were reported over the study period (95% CI 8.6–9.6) (Table A1). Compared with all other reported conditions, higher rates of disease were reported for the following chapters (in order of highest to lowest RR): endocrine, nutritional, and metabolic diseases (RR 5.3, 95% CI 4.2–6.6); neoplasms (RR 4.9, 95% CI 4.4–5.5); mental and behavior disorders (RR 3.1, 95% CI 2.4–4.1); diseases of the circulatory system (RR 1.4, 95% CI 1.2–1.6); diseases of the digestive system (RR 1.3, 95% CI 1.1–1.5); and diseases of the musculoskeletal system and connective tissue (RR 1.3, 95% CI 1.1–1.6) (Table 2).
Within these chapters, only certain subgroups showed increased rates: diabetes mellitus; malignant neoplasms of the lymphoid, hematopoietic, and related tissues; eye, brain, and other parts of the central nervous system (CNS); respiratory and intrathoracic organs; digestive organs; breast; male and female genital organs; thyroid and other endocrine glands; mental and behavior disorders caused by psychoactive substances (alcohol-related in 96% of reports); hypertensive diseases, other forms of heart disease, and diseases of arteries, arterioles, and capillaries; diseases of the liver and noninfective enteritis and colitis; and systemic connective tissue disorders (Table 2). In addition, several subgroups were associated with increased risk even when the corresponding chapter was not: renal failure, diseases of blood and blood-forming organs, and chronic lower respiratory diseases (Table 2).
Concurrent conditions were disproportionately reported for persons >60 years of age (χ2 p<0.001), and the rate of listeriosis for this age group (16.8/million; 95% CI 15.8–17.9) was significantly higher than that for younger persons (RR 4.6, 95% CI 4.1–5.3) (Table 2). When the RR for each chapter for persons >60 years of age (using persons <60 years of age as the reference population) was calculated, the following were associated with increased risk: endocrine, nutritional and metabolic diseases; genitourinary system diseases; diseases of the musculoskeletal system and connective tissue; neoplasms; certain infectious and parasitic diseases; diseases of the digestive system; and mental and behavior disorders (Table 2). In instances where the risk for each subgroup in persons >60 years of age could be calculated and compared with that for persons <60 years of age, all subgroups of previously identified chapters were associated with increased risk.
We analyzed surveillance data that included detailed denominator data by using an internationally recognized diagnostic classification system and found that a wide variety of conditions seem to increase the risk for serious infection with L. monocytogenes. Malignancies accounted for more than one third of conditions, and cancer patients had a 5-fold increased risk for development of listeriosis. Cancers of the blood seemed to have the greatest effect. Other high-risk conditions included diabetes mellitus; alcoholism; certain diseases of the circulatory system and the musculoskeletal system and connective tissue; noninfective enteritis and colitis; and diseases of the liver and kidney. For most high-risk conditions, the risk for infection was higher among older patients.
Case identified by the national surveillance program in England are laboratory confirmed, and most cases result in serious illness requiring hospitalization or death. Given this finding, a hospitalized population better represents the population at risk than a community population, which was used in previous studies (10,11).
The response rate to the clinical questionnaire that captured information on concurrent conditions was high and not influenced by age or sex of the case-patient, which minimized differential ascertainment of clinical data. However, we could not assess concurrent conditions for which completed clinical questionnaires were not returned. This issue indicates that the role of some conditions might be underestimated if clinicians were unwilling to return questionnaires and disclose information for certain case-patients (e.g., those with AIDS). Similarly, but less likely, reporting bias might exist if the propensity to report certain concurrent conditions were affected by the presence or absence of others conditions, or if only concurrent conditions considered relevant to L. monocytogenes infection were reported. Concurrent conditions were reported by the clinical microbiologist rather than by the consultants responsible for the care of the patients with concurrent conditions. These consultants might be better informed of existing concurrent conditions. However, hospital microbiologists need to be aware of such conditions to provide treatment accordingly, and questioning several consultants for each case-patient may have a negative effect on questionnaire response because questionnaires might be lost if passed between multiple consultants.
Misclassification was minimized by grouping conditions only to 3-character ICD-10 code levels. Although we acknowledge that such grouping might mask high-risk conditions apparent at the 4-character ICD-10 code level, routine surveillance data were not specific enough to further discriminate among conditions. In some instances, in which treatments were reported in the absence of relevant conditions (e.g., chemotherapy, dialysis, splenectomy), we made assumptions about the conditions requiring such treatment and coded accordingly (Table A1). Although these assumptions could inflate the incidence rates for certain conditions, they occurred relatively infrequently and were not used for treatments that could be prescribed for a range of conditions (e.g., broad-spectrum antimicrobial drugs).
Because only single-variable analysis could be performed, we could not assess the extent to which concurrent conditions were correlated, which led to the potential for uncontrolled confounding. Such method limitations might explain the high incidence associated with both diabetes and kidney disease and reinforce the need to consider these findings as highly refined hypotheses to be tested by other methods (12).
To our knowledge, few studies have attempted to quantify the risk for listeriosis by patient concurrent conditions. As part of a risk assessment of L. monocytogenes in ready-to-eat foods, researchers from the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) calculated the relative susceptibility to listeriosis for certain conditions (10). Furthermore, risk levels for listeriosis by predisposing condition in Denmark have also been estimated (11). Despite differences in methods between those studies and our study, several high-risk conditions were also identified in those studies: malignancies (most notably those of the blood), kidney disease (recorded as dialysis [10] and renal transplant [11]), diabetes, alcoholism, and increased age in all 3 studies; liver disease and pulmonary cancer in the WHO/FAO study and our study; and systemic lupus erythematosus in the study in Denmark and our study (as systemic connective tissue disorders). Such commonality would seemingly validate our estimates.
The absence of AIDS as a high-risk condition in our study and its presence in both previous studies (10,11), might reflect improved treatment for HIV infection that prevents AIDS and, consequently, L. monocytogenes infection (13) or highlight a reporting bias by the consultant microbiologist. A general transplantation status, identified as a condition leading to the highest relative susceptibility in the WHO/FAO study, was not coded in our study because it is a treatment. Noninfective enteritis and colitis and certain diseases of the circulatory system were identified as additional high-risk conditions in our study but not in the previous studies. These additional conditions might be the result of improved accuracy, use of ICD-10 coding and a hospitalized reference population instead of the general population, different susceptibility calculations, or changes in the prevalence of certain conditions in the interim period (the previous studies used data from 1992 [10] and 1989–1990 [11]). However, we acknowledge that links between these conditions and listeriosis have been reported (14–18).
With these caveats in mind, our findings have implications for clinical practice and food safety policy makers. The number and diversity of conditions that appear to increase the risk for listeriosis imply that physicians working in all specialties should consider listeriosis when treating patients with concurrent conditions and provide appropriate food safety advice. Similarly, current UK government food safety advice on avoidance of listeriosis, which is delivered passively and is specific mainly for pregnant women (19,20), should be communicated actively to all high-risk groups. In prioritizing advice, policy makers should consider not only the associated risk but also the prevalence of the concurrent condition. Cancer patients accounted for more than one third of listeriosis cases, and high risks were observed for most cancer subgroups. Because we are not aware of any appropriate food safety advice that is tailored specifically for cancer patients in the UK, emphasis on this group might help to prevent further cases.
Mr Mook is a scientist at the Health Protection Agency in London. His research interests are seasonal influenza surveillance, preparation for pandemic influenza, and surveillance and outbreak response for listeriosis.
Acknowledgment
We thank the hospital microbiologists, environmental health officers, and public health professionals for contributing to the surveillance system; Northgate Public Services for supporting the Hospital Episodes Statistics data; George Kafatos for providing statistical consultations; and Christine Little for assisting in preparation of the manuscript.
References
- Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511.PubMedGoogle Scholar
- Gillespie IA, McLauchlin J, Grant KA, Little CL, Mithani V, Penman C, Changing pattern of human listeriosis, England and Wales, 2001–2004. Emerg Infect Dis. 2006;12:1361–6.PubMedGoogle Scholar
- Goulet V, Hedberg C, Le Monnier A, de Valk H. Increasing incidence of listeriosis in France and other European countries. Emerg Infect Dis. 2008;14:734–40. DOIPubMedGoogle Scholar
- Koch J, Stark K. Significant increase of listeriosis in Germany—epidemiological patterns 2001–2005. Euro Surveill. 2006;11:85–8.PubMedGoogle Scholar
- Kvistholm JA, Ethelberg S, Smith B, Moller NE, Larsson J, Molbak K, Substantial increase in listeriosis, Denmark 2009. Euro Surveill. 2010;15:pii: 19522.
- Gillespie IA, McLauchlin J, Little CL, Penman C, Mook P, Grant K, Disease presentation in relation to infection foci for non-pregnancy-associated human listeriosis in England and Wales, 2001 to 2007. J Clin Microbiol. 2009;47:3301–7. DOIPubMedGoogle Scholar
- World Health Organization. International statistical classification of diseases and related health problems, 10th revision; 2007 [cited 2009 Mar 6]. http://apps.who.int/classifications/apps/icd/icd10online
- Hospital Episode Statistics (HES) Online [cited 2009 Feb 23]. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=202
- World Health Organization. Risk assessment of Listeria monocytogenes in ready-to-eat foods. Microbiological risk assessment series 5; 2004 [cited 20101 Oct 18]. http://www.who.int/foodsafety/publications/micro/en/mra5_contents.pdf
- Jensen A, Frederiksen W, Gerner-Smidt P. Risk factors for listeriosis in Denmark, 1989–1990. Scand J Infect Dis. 1994;26:171–8. DOIPubMedGoogle Scholar
- Gradel KO, Schonheyder HC, Kristensen B, Dethlefsen C, Ejlertsen T, Nielsen H. Are host characteristics or exposure factors mainly involved in the acquisition of zoonotic Salmonella and Campylobacter coinfection in humans? Foodborne Pathog Dis. 2009;6:251–5. DOIPubMedGoogle Scholar
- Bennion JR, Sorvillo F, Wise ME, Krishna S, Mascola L. Decreasing listeriosis mortality in the United States, 1990–2005. Clin Infect Dis. 2008;47:867–74. DOIPubMedGoogle Scholar
- Dalton CB, Merritt TD, Unicomb LE, Kirk MD, Stafford D, Lalor K. the OzFoodNet Working Group. A national case-control study of risk factors for listeriosis in Australia. [Epub ahead of print]. Epidemiol Infect. 2010;1–9.
- Goulet V, Marchetti P. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scand J Infect Dis. 1996;28:367–74. DOIPubMedGoogle Scholar
- McLauchlin J. Human listeriosis in Britain, 1967–85, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol Infect. 1990;104:191–201. DOIPubMedGoogle Scholar
- Nieman RE, Lorber B. Listeriosis in adults: a changing pattern. Report of eight cases and review of the literature, 1968–1978. Rev Infect Dis. 1980;2:207–27. DOIPubMedGoogle Scholar
- Siegman-Igra Y, Levin R, Weinberger M, Golan Y, Schwartz D, Samra Z, Listeria monocytogenes infection in Israel and review of cases worldwide. Emerg Infect Dis. 2002;8:305–10.PubMedGoogle Scholar
- Food Standards Agency. Eat well, be well. Pregnancy [cited 2009 Sep 15]. http://www.eatwell.gov.uk/asksam/agesandstages/pregnancy
- Food Standards Agency. Eat well, be well. Look out for Listeria [cited 2010 Oct 21]. http://eatwell.gov.uk/healthissues/foodpoisoning/listeria/whatisit
Figure
Tables
Cite This ArticleTable of Contents – Volume 17, Number 1—January 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Piers Mook, Gastrointestinal, Emerging and Zoonotic Infections Department, Centre for Infections, Health Protection Agency, 61 Colindale Ave, London NW9 5EQ, UK
Top