Volume 24, Number 8—August 2018
CME ACTIVITY - Synopsis
Epidemiology of Diphyllobothrium nihonkaiense Diphyllobothriasis, Japan, 2001–2016
Identification of Etiologic Agents of Tapeworm Infections
D. nihonkaiense Diphyllobothriasis Annual and Seasonal Occurrence
Geographic Distribution of D. nihonkaiense Diphyllobothriasis
Demographic Analysis of Patients and Clinical Signs
Possible Sources and Locality of Infection
Treatment and Prevention
Perspectives of Diphyllobothriasis
Conclusions
CME Follow Up
Cite This Article
Introduction

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; and (4) view/print certificate.
Release date: July 13, 2018; Expiration date: July 13, 2019
Learning Objectives
Upon completion of this activity, participants will be able to:
• Assess the parasitology of diphyllobothriosis
• Analyze the epidemiology of diphyllobothriosis
• Evaluate the clinical presentation of diphyllobothriosis
• Distinguish the most common treatment for diphyllobothriosis
CME Editor
Kristina B. Clark, PhD, Copyeditor, Emerging Infectious Diseases. Disclosure: Kristina B. Clark, PhD, has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, FAAFP, Health Sciences Clinical Professor, University of California, Irvine, Department of Family Medicine; Associate Dean for Diversity and Inclusion, University of California, Irvine, School of Medicine; Executive Director, University of California, Irvine, Program in Medical Education for the Latino Community, Irvine, California. Disclosure: Charles P. Vega, MD, FAAFP, has disclosed the following relevant financial relationships: served as an advisor or consultant for Johnson & Johnson Healthcare; served as a speaker or a member of a speakers bureau for Shire Pharmaceuticals.
Authors
Disclosures: Hiroshi Ikuno, BS; Shinkichi Akao, PhD; and Hiroshi Yamasaki, PhD, have disclosed no relevant financial relationships.
Abstract
We report 958 cases of cestodiasis occurring in Japan during 2001–2016. The predominant pathogen was Diphyllobothrium nihonkaiense tapeworm (n = 825), which caused 86.1% of all cases. The other cestode species involved were Taenia spp. (10.3%), Diplogonoporus balaenopterae (3.3%), and Spirometra spp. (0.2%). We estimated D. nihonkaiense diphyllobothriasis incidence as 52 cases/year. We observed a predominance of cases during March–July, coinciding with the cherry salmon and immature chum salmon fishing season, but cases were present year-round, suggesting that other fish could be involved in transmission to humans. Because of increased salmon trade, increased tourism in Japan, and lack of awareness of the risks associated with eating raw fish, cases of D. nihonkaiense diphyllobothriasis are expected to rise. Therefore, information regarding these concerning parasitic infections and warnings of the potential risks associated with these infections must be disseminated to consumers, food producers, restaurant owners, physicians, and travelers.
In Japan, the occurrence of soil-transmitted helminthiases declined sharply in 1949 (1). However, foodborne parasitic infections, which are closely associated with the Japanese food custom of eating raw fish, have remained. Diphyllobothriasis caused by the adult tapeworm Diphyllobothrium nihonkaiense (proposed as Dibothriocephalus nihonkaiensis in 2017) (2), an infection closely associated with the consumption of raw Pacific salmon, is the most frequently occurring foodborne parasitic infection in Japan. Paleoparasitologic studies have revealed that diphyllobothriasis has existed in Japan for ≈1,000 years (3).
Adult D. nihonkaiense tapeworms are ribbon-like and composed of a slender and spatulated scolex (2.4–2.8-mm long and 1.2–1.5-mm wide) with paired slit-like bothria, neck (14.4–16.8-mm long and 1.16–1.28-mm wide), and strobila comprising numerous proglottids (4) (Figure 1). The D. nihonkaiense tapeworm is parasitic in mammals; brown bear, domestic dog, and humans are their definitive hosts (5,6). Inside humans, the parasite can grow >10 m in length. Adult worms lay millions of eggs, and these eggs are excreted in feces. The D. nihonkaiense tapeworm, as well as other diphyllobothriid species, uses 2 intermediate hosts to complete its life cycle (4–6). The first intermediate host (species in which the procercoid develops) is probably brackish zooplanktonic copepods (7). The first intermediate host is consumed by the second intermediate host, Pacific salmonids, namely cherry salmon (Oncorhynchus masou), chum salmon (O. keta), and pink salmon (O. gorbuscha) (8–10). In the second intermediate host, procercoids develop into plerocercoids, the larval form needed to infect the definitive host (e.g., humans). D. nihonkaiense infections are generally asymptomatic or induce relatively mild symptoms, such as mild diarrhea and abdominal pain (5,6,11).
In Japan, the causative agent of diphyllobothriasis has long been considered to be the tapeworm Diphyllobothrium latum (proposed as Dibothriocephalus latus in 2017) (2), ever since the first case of diphyllobothriasis reported in 1889 (12). This belief has caused confusion over diagnostics; whether the cases of diphyllobothriasis reported in Japan in the past were caused by D. latum tapeworm or another species was debatable (13). However, in 1986, Yamane et al. (4) identified the causative agent of diphyllobothriasis as the D. nihonkaiense tapeworm from Japan, which is morphologically and ecologically distinct from the D. latum tapeworm from Finland. This finding was further verified by DNA analyses (14–16).
In Japan, all infections, including parasitic infections, linked to the consumption of food should be reported to health authorities as food poisoning, in accordance with the Ordinance for Enforcement of the Food Sanitation Act of 2012. However, despite diphyllobothriasis being the most frequent parasitic infection in Japan, no cases have been duly reported. Thus, diphyllobothriasis epidemiology has been estimated by using only case reports published in journals and the number of outpatients in hospitals (5,17).
The Department of Bacteriology of BML Inc. (Kawagoe, Saitama, Japan) routinely identifies parasites and diagnoses parasitic infections as requested by physicians from the medical institutions of Japan. During 2001–2016, we examined 632 proglottid samples and 326 egg samples from 958 patients with cestodiasis (Table). In this article, we report the etiologic agents associated with cestodiasis, focusing on diphyllobothriasis, the predominant type of cestodiasis in Japan. We describe the geographic distribution of D. nihonkaiense diphyllobothriasis cases and demographic characteristics of patients with this infection. Perspectives of diphyllobothriasis are also discussed.
Proglottid and egg samples were collected from patients with diphyllobothriasis and taeniasis in hospitals in Japan. Proglottids were fixed in formalin solution by hospital staff and sent to BML Inc.’s general laboratory for species identification. Almost all proglottids were not attached to a scolex; only 26 (20 diphyllobothriids and 6 taeniids) proglottids had a scolex attached. Fecal samples containing eggs were also collected by hospital staff and sent to BML Inc. These samples were not fixed with formalin; the eggs in the fecal samples were concentrated, and the species were identified on the basis of morphology and egg size. Two Spirometra plerocercoids removed surgically from a subcutaneous nodule in the abdomen of 1 patient and a subcutaneous nodule in the ankle of another patient were also received as formalin-fixed samples. We identified proglottids by their morphologic and morphometric markers, such as length and width of mature proglottids and ratio, number, shape, and position of the hermaphrodite genitalia; we also noted the shape and size of the scolex (if available) and the eggs in the uterus (4).
During 2012–2016, we identified 179 proglottid samples (161 diphyllobothriids and 18 taeniids) using molecular methods (Table); restriction fragment length polymorphism analysis with PCR-amplified cytochrome c oxidase subunit 1 (cox1) gene fragment (249-bp long corresponding to base pairs 880–1128) was introduced to confirm diphyllobothriid species (18–20) and PCR-amplified cox1 sequencing (145-bp long corresponding to base pairs 641–785) was used for identification of taeniid species (20). To amplify the cox1 gene fragments, we used paired primers 5′-ACAGTGGGTTTAGATGTAAAGACGGC-3′ (forward) and 5′-AGCTACAACAAACCAAGTATCATG-3′ (reverse) for diphyllobothriids (19) and 5′-AATTTAGTTCTGCGTTTTTTTGATCC-3′ (forward) and 5′-CTTATWCTRAAACATATATGACTAAT-3′ (reverse) for taeniids (20).
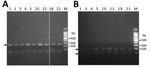
Of the 958 cestode samples we examined, 825 (526 proglottid and 299 egg, 86.1%) were D. nihonkaiense, 32 (3.3%) were Diplogonoporus balaenopterae (proposed as Diphyllobothrium balaenopterae in 2017) (2), 2 (0.2%) were Spirometra spp., and 99 (10.3%) were Taenia spp. (Table). Of the 179 diphyllobothriid proglottids with which we performed restriction fragment length polymorphism, 153 were confirmed as D. nihonkaiense (Figure 2) and 8 as Dip. balaenopterae. Of the 18 taeniid proglottids we tested by cox1 sequencing, 16 were Taenia saginata, 1 was T. solium, and 1 was T. asiatica.
Regarding Spirometra plerocercoids, 2 species (S. erinaceieuropaei and S. decipiens) have been found to be responsible for human sparganosis in Japan (21). However, these 2 species were not identified by DNA analysis in this study.
We analyzed the 825 diphyllobothriasis cases attributed to D. nihonkaiense infection for their annual and seasonal occurrence. D. nihonkaiense diphyllobothriasis occurred persistently, although the frequency varied over the years of the study (Figure 3). Using our data, we estimated that 52 D. nihonkaiense diphyllobothriasis cases occurred per year in Japan. The rate of D. nihonkaiense diphyllobothriasis cases estimated by examining reports in the literature was ≈40 cases/year (17). However, the actual rate is probably much higher and has been estimated to be 100–200 cases/year (17).
Although D. nihonkaiense diphyllobothriasis occurred throughout the year, the incidence was remarkably higher during March–July, showing a seasonal pattern of occurrence (Technical Appendix Figure 1). Considering that the prepatent period (time from start of infection to time infection is discovered, e.g., person notices strobila excreted in feces) is 2–4 weeks, patients probably acquired infective plerocercoids during February–June. This timing coincides with the season when cherry salmon and immature chum salmon are usually caught and sold. Although specifying the sources of infection is difficult, Pacific salmon are clearly implicated; cherry salmon are caught during March–May, and tokishirazu (i.e., immature chum salmon), which originate from the Amur River in Russia, are caught during May–July (9). D. nihonkaiense plerocercoids have not been found in akizake (i.e., mature chum salmon), which are not prevalent in fishing waters during February–June because they return to their natal rivers for spawning in autumn (9). However, considering that D. nihonkaiense diphyllobothriasis occurs throughout the year, akizake and other fish that salmonids eat might also be associated with the occurrence of diphyllobothriasis. Further study is necessary for elucidating this possibility.
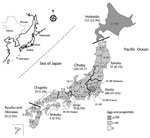
D. nihonkaiense diphyllobothriasis occurred widely (40/47 prefectures) throughout Japan, from Hokkaido Prefecture to Okinawa Prefecture. The regions where D. nihonkaiense diphyllobothriasis occurred most often were the populous cities of Tokyo and Saitama in the Kanto region (Figure 4), owing to their high consumption of raw Pacific salmon, followed by the Hokkaido Prefecture, Chubu region along the Sea of Japan, Tohoku region (where salmon are caught and consumed locally), and Kinki region (with populous prefectures, e.g., Osaka Prefecture). The incidence of D. nihonkaiense diphyllobothriasis was lower in the southern regions than in the northern regions.
From 1979 through the 1990s, diphyllobothriasis occurred mainly in Hokkaido Prefecture, Tohoku region, and along the coastal regions of the Sea of Japan, where salmon are caught and consumed locally (22). However, with the rapid advancement of food transportation systems and techniques to retain freshness, diphyllobothriasis spread from the northern parts of Japan to its big cities, such as Tokyo and Osaka, in which salmon consumption has been on the rise since the 1990s.
During 2012–2015, we conducted a survey to investigate patient demographics. Of the 139 patients who participated, 136 indicated their sex: 85 (61%) were male and 51 (37%) were female. In total, 114 patients indicated their age; age ranged from 2 years to >90 years, but most patients (of either sex) were in the 20–60-year age range (Technical Appendix Figure 2).
Most patients noticed they expelled strobilae when they defecated; for 8 patients, the strobilae were incidentally detected during colonoscopy. Of the 78 patients indicating clinical symptoms, 29 (37.1%) were asymptomatic. Light diarrhea occurred in 28 (34.0%) patients; abdominal pain in 18 (22.0%) patients; abdominal discomfort in 4 (4.9%) patients; and constipation, vomiting, and weight loss in 1 patient each. Most patients experienced mental distress over defecating and discharging proglottids.
Regarding questions on the consumption of raw fish in the 2012–2015 patient survey, 12 of 15 patients replied that they had eaten dishes containing raw salmon, such as sushi and sashimi. However, the salmon species consumed could not be specified in all cases.
Seven patients had traveled abroad, some to multiple countries: 4 patients went to the United States; 2 patients to South Korea; and 1 patient each to Vietnam, Myanmar, the Netherlands, Belgium, and Italy. However, all 7 patients were considered to have been infected with D. nihonkaiense tapeworm in Japan because they had not consumed any kind of raw fish during travel.
The patients with diplogonoporiasis caused by Dip. balaenopterae infection were also all infected in Japan. The 17 cases of T. saginata and T. solium infection that occurred during 2012–2016 were all imported cases, but 1 case of T. asiatica infection was acquired in Japan through the consumption of raw pork liver.
Praziquantel is recommended as the first-choice anthelminthic drug for diphyllobothriasis (23), and this drug was used in all the cases in this study. To prevent recurrence, excretion of the scolex in the feces must be confirmed. If excretion is not detected, further observation of the feces for discharged proglottids or eggs is needed for 2–3 more months.
The most effective prevention method for diphyllobothriasis is to avoid the consumption of raw and undercooked Pacific salmon. If the salmon is cooked at 55°C or frozen at either −8°C for 12 hours or −10°C for 6 hours, plerocercoids in the salmon are killed, and their infectivity is lost (24). The US Food and Drug Administration recommends that fish be frozen at −35°C for 15 hours or −20°C for 7 days before consumption of raw or poorly cooked fish (25). However, this standard is difficult to achieve in Japan, considering the preference for and, thus, high consumption of traditional raw fish dishes.
In Japan, deep freezing has become a legal obligation to prevent infection with Kudoa septempunctata myxozoan parasite in flounder and Sarcocystis fayeri protozoon parasite in horse meat. However, this practice has not been implemented for the fishborne parasites Diphyllobothrium spp., Dibothriocephalus spp., and Anisakis spp.
The number of diphyllobothriasis cases attributable to D. nihonkaiense infection is expected to rise in Japan, considering this pathogen’s association with Japanese food customs. Also on the rise in Japan is tourism; in 2016, ≈24 million international travelers came to Japan, a 21.8% increase from the year before (http://www.jnto.go.jp/jpn/statistics/visitor_trends/index.html). With the increase in numbers of persons traveling to Japan for sightseeing and business purposes, international travelers acquiring infections with D. nihonkaiense tapeworm via the consumption of Japanese foods made with raw salmon, such as sushi and sashimi, is of great concern. In fact, 1 case was reported in a visitor from China (26).
Infection with D. nihonkaiense tapeworm is no longer a public health problem limited to East Asia and the North Pacific coast of North America; this pathogen is spreading due to the globalization of trade and increased commerce with salmon. Several cases of infection with D. nihonkaiense tapeworm have been reported in Europe (27) and New Zealand (28), where this pathogen was previously absent. The sources of infection for these cases are suspected to be the salmon imported from North America (27). Furthermore, regardless of immunity, anyone can get infected with D. nihonkaiense tapeworm in the countries where the pathogen exists, such as Korea (29,30), China (26,31), the United States (32), Canada (33,34), and eastern Russia (35).
Besides infections with D. nihonkaiense tapeworm, the following rare and autochthonous cestodes have been sporadically reported in humans in Japan: Diphyllobothrium stemmacephalum (36), Adenocephalus pacificus (37), Dip. balaenopterae (38), and Spirometra spp. (39). In contrast, human taeniasis has been exclusively reported as imported cases, but T. asiatica infections in Japan have been confirmed to be autochthonous infections through the consumption of raw pork liver (17,40).
From the public health point of view, most of the population in Japan are still unaware of the risk for D. nihonkaiense infection associated with the consumption of raw salmon or the risks for infections with other cestodes. Therefore, information regarding parasitic infections and warnings of the potential risks associated with these infections must be disseminated to consumers, food producers, restaurant owners, physicians, and visitors.
D. nihonkaiense diphyllobothriasis is no longer a public health issue limited to only East Asia, including Japan, and North America but is becoming a global threat due to the increasing consumption of raw salmon worldwide. Since 2005, D. nihonkaiense diphyllobothriasis has been reported in Europe and New Zealand, where the disease has no endemic foci. Considering these global occurrences, anyone consuming salmon is at risk for D. nihonkaiense diphyllobothriasis, not only in Japan and along the North Pacific coast of North America, where local salmon is consumed, but also in Europe, where imported salmon is consumed. The effects of globalization, such as the expansion of the salmon market, the increase in travel to and from diphyllobothriasis-endemic countries, and the global change in eating habits, might cause an increase in the incidence of D. nihonkaiense infections worldwide, in places where diphyllobothriasis was previously present and in places where it was not.
Mr. Ikuno is a medical technologist at the Department of Bacteriology, BML Inc., Kawagoe, Saitama, Japan. His research interests are the epidemiology of foodborne parasitic zoonoses.
Acknowledgments
The authors thank the staff members of the domestic medical institutions who participated in the survey.
This study was partially supported by grants-in-aid from the Ministry of Health, Labour and Welfare, Japan (H24-H26 Shinkosaiko-Ippan-016, to H.Y.), and the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (H27-H28 Kansensho-Jitsuyouka-Ippan, 40100703, to H.Y.).
References
- Kobayashi A, Hara T, Kajima J. Historical aspects for the control of soil-transmitted helminthiases. Parasitol Int. 2006;55(Suppl):S289–91. DOIPubMedGoogle Scholar
- Waeschenbach A, Brabec J, Scholz T, Littlewood DTJ, Kuchta R. The catholic taste of broad tapeworms - multiple routes to human infection. Int J Parasitol. 2017;47:831–43. DOIPubMedGoogle Scholar
- Matsui A, Kanehara M, Kanehara M. Palaeoparasitology in Japan—discovery of toilet features. Mem Inst Oswaldo Cruz. 2003;98(Suppl 1):127–36. DOIPubMedGoogle Scholar
- Yamane Y, Kamo H, Bylund G, Wikgren BJP. Diphyllobothrium nihonkaiense sp. nov. (Cestoda: Diphyllobothriidae): revised identification of Japanese broad tapeworm. Shimane J Med Sci. 1986;10:29–48.
- Arizono N, Yamada M, Nakamura-Uchiyama F, Ohnishi K. Diphyllobothriasis associated with eating raw pacific salmon. Emerg Infect Dis. 2009;15:866–70. DOIPubMedGoogle Scholar
- Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146–60. DOIPubMedGoogle Scholar
- Muratov IV. [A new type of diphyllobothriasis foci in the Far East] [in Russian]. Med Parazitol (Mosk). 1992;61:25–7.PubMedGoogle Scholar
- Ando K, Ishikura K, Nakakugi T, Shimono Y, Tamai T, Sugawa M, et al. Five cases of Diphyllobothrium nihonkaiense infection with discovery of plerocercoids from an infective source, Oncorhynchus masou ishikawae. J Parasitol. 2001;87:96–100. DOIPubMedGoogle Scholar
- Suzuki J, Murata R, Sadamasu K, Araki J. Detection and identification of Diphyllobothrium nihonkaiense plerocercoids from wild Pacific salmon (Oncorhynchus spp.) in Japan. J Helminthol. 2010;84:434–40. DOIPubMedGoogle Scholar
- Kuchta R, Oros M, Ferguson J, Scholz T. Diphyllobothrium nihonkaiense tapeworm larvae in salmon from North America. Emerg Infect Dis. 2017;23:351–3. DOIPubMedGoogle Scholar
- Tsuboi M, Hayakawa K, Yamasaki H, Katanami Y, Yamamoto K, Kutsuna S, et al. Clinical characteristics and epidemiology of intestinal tapeworm infections over the last decade in Tokyo, Japan: A retrospective review. PLoS Negl Trop Dis. 2018;12:e0006297. DOIPubMedGoogle Scholar
- Iijima I. The source of Bothricephaus latus in Japan. J Coll Sci Tokyo Imp Univ. 1889;2:49–56.
- Dick TA, Nelson PA, Choudhury A. Diphyllobothriasis: update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian J Trop Med Public Health. 2001;32(Suppl 2):59–76.PubMedGoogle Scholar
- Nakao M, Abmed D, Yamasaki H, Ito A. Mitochondrial genomes of the human broad tapeworms Diphyllobothrium latum and Diphyllobothrium nihonkaiense (Cestoda: Diphyllobothriidae). Parasitol Res. 2007;101:233–6. DOIPubMedGoogle Scholar
- Kim KH, Jeon HK, Kang S, Sultana T, Kim GJ, Eom K, et al. Characterization of the complete mitochondrial genome of Diphyllobothrium nihonkaiense (Diphyllobothriidae: Cestoda), and development of molecular markers for differentiating fish tapeworms. Mol Cells. 2007;23:379–90.PubMedGoogle Scholar
- Park JK, Kim KH, Kang S, Jeon HK, Kim JH, Littlewood DT, et al. Characterization of the mitochondrial genome of Diphyllobothrium latum (Cestoda: Pseudophyllidea) - implications for the phylogeny of eucestodes. Parasitology. 2007;134:749–59. DOIPubMedGoogle Scholar
- Yamasaki H, Morishima Y, Sugiyama H. Current status of cestodioses in Japan [In Japanese]. Infect Agents Surveill Rep. 2017;38:74–6.
- Yamasaki H, Nakaya K, Nakao M, Sako Y, Ito A. Significance of molecular diagnosis using histopathological specimens in cestode zoonoses. Trop Med Health. 2007;35:307–21. DOIGoogle Scholar
- Yamasaki H, Tsubokawa D, Mercado R, Kuramochi T. A simple method for identifying the diphyllobothriids based on mitochondrial DNA analysis. In: Takamiya S, editor. Materials and methods in parasitology [in Japanese]. Nagoya City (Japan): Sankeisha; 2014. p. 47–9.
- Yamasaki H, Morishima Y, Sugiyama H. Molecular identification of human taeniids based on mitochondrial DNA analysis. In: Takamiya S, editor. Materials and methods in parasitology [in Japanese]. Nagoya City (Japan): Sankeisha; 2014. p. 73–6.
- Yamasaki H, Morishima Y, Sugiyama H, Korenaga M, Eom KS. Molecular evidence of Spirometra species causing human sparganosis in Japan [in Japanese]. Clin Parasitol. 2017;28:99–102.DOIGoogle Scholar
- Kagei N. Existence of Diphyllobothrium nihonkaiense and its occurrence in Japan [in Japanese]. Infect Agents Surveill Rep. 1993;14:16106.
- Ohnishi K, Kato Y. Single low-dose treatment with praziquantel for Diphyllobothrium nihonkaiense infections. Intern Med. 2003;42:41–3. DOIPubMedGoogle Scholar
- Eguchi S. Diphyllobothrium latum (Linnaeus, 1758). In: Morishita K, Komiya Y, Matsubayashi H, editors. Progress of medical parasitology in Japan. Vol. 5. Tokyo: Meguro Parasitological Museum; 1973. p. 127–44.
- US Food and Drug Administration. Fish and fisheries products hazards and controls guide, 2nd ed. Washington: The Administration; 1998.
- Chen S, Ai L, Zhang Y, Chen J, Zhang W, Li Y, et al. Molecular detection of Diphyllobothrium nihonkaiense in humans, China. Emerg Infect Dis. 2014;20:315–8. DOIPubMedGoogle Scholar
- de Marval F, Gottstein B, Weber M, Wicht B. Imported diphyllobothriasis in Switzerland: molecular methods to define a clinical case of Diphyllobothrium infection as Diphyllobothrium dendriticum, August 2010. Euro Surveill. 2013;18:20355.PubMedGoogle Scholar
- Yamasaki H, Kuramochi T. A case of Diphyllobothrium nihonkaiense infection possibly linked to salmon consumption in New Zealand. Parasitol Res. 2009;105:583–6. DOIPubMedGoogle Scholar
- Kim HJ, Eom KS, Seo M. Three cases of Diphyllobothrium nihonkaiense infection in Korea. Korean J Parasitol. 2014;52:673–6. DOIPubMedGoogle Scholar
- Go YB, Lee EH, Cho J, Choi S, Chai JY. Diphyllobothrium nihonkaiense infections in a family. Korean J Parasitol. 2015;53:109–12. DOIPubMedGoogle Scholar
- Zhang W, Che F, Tian S, Shu J, Zhang X. Molecular identification of Diphyllobothrium nihonkaiense from 3 human cases in Heilongjiang Province with a brief literature review in China. Korean J Parasitol. 2015;53:683–8. DOIPubMedGoogle Scholar
- Fang FC, Billman ZP, Wallis CK, Abbott AN, Olson JC, Dhanireddy S, et al. Human Diphyllobothrium nihonkaiense infection in Washington State. J Clin Microbiol. 2015;53:1355–7. DOIPubMedGoogle Scholar
- Wicht B, Scholz T, Peduzzi R, Kuchta R. First record of human infection with the tapeworm Diphyllobothrium nihonkaiense in North America. Am J Trop Med Hyg. 2008;78:235–8.PubMedGoogle Scholar
- Cai YC, Chen SH, Yamasaki H, Chen JX, Lu Y, Zhang YN, et al. Four human cases of Diphyllobothrium nihonkaiense (Eucestoda: Diphylloboyhriidae) in China with a brief review of Chinese cases. Korean J Parasitol. 2017;55:319–25. DOIPubMedGoogle Scholar
- Muratov IV. Diphyllobothriasis in the far east of the USSR [in Russian]. Med Parazitol (Mosk). 1990; (
6 ):54–8.PubMedGoogle Scholar - Yamasaki H, Kumazawa H, Sekikawa Y, Oda R, Hongo I, Tsuchida T, et al. First confirmed human case of Diphyllobothrium stemmacephalum infection and molecular verification of the synonymy of Diphyllobothrium yonagoense with D. stemmacephalum (Cestoda: Diphyllobothriidea). Parasitol Int. 2016;65(5 Pt A):412–21. DOIPubMedGoogle Scholar
- Yamane Y, Shiwaku K. Diphyllobothrium nihonkaiense and other marine-origin cestodes. In: Otsuru M, Kamegai S, Hayashi S, editors. Progress of medical parasitology in Japan. Vol 8. Tokyo: Meguro Parasitological Museum; 2003. p. 245–59.
- Kawai S, Ishihara Y, Sasai T, Takahashi F, Kirinoki M, Kato-Hayashi N, et al. A case of cestode infection caused by Diplogonoporus balaenopterae most likely due to the consumption of raw whitebait [in Japanese]. Dokkyo J Med Sci. 2013;40:189–92.
- Kudo T, Fujioka A, Korenaga M, Yamasaki H, Morishima Y, Sugiyama H, et al. Molecular identification of intramuscular and subcutaneous Spirometra erinaceiuropaei sparganosis in a Japanese patient. J Dermatol. 2017;44:e138–9. DOIPubMedGoogle Scholar
- Yamasaki H. Current status and perspectives of cysticercosis and taeniasis in Japan. Korean J Parasitol. 2013;51:19–29. DOIPubMedGoogle Scholar
Figures
Table
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on http://www.medscape.org. If you are not registered on http://www.medscape.org, please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@medscape.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to https://www.ama-assn.org. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Article Title:
Epidemiology of Diphyllobothrium nihonkaiense Diphyllobothriasis, Japan, 2001–2016
CME Questions
1. You are seeing a 30-year-old woman who complains of having diarrhea and abdominal pain for 2 weeks. She has attended an "all you can eat" sushi and sashimi night at a Japanese restaurant for years, but she does not believe that has anything to do with her present symptoms. You wonder whether she has diphyllobothriosis. What should you consider regarding the parasitology of this infection?
A. Diphyllobothriosis is closely associated with the consumption of raw Pacific salmon
B. The only mammals to be infected with Diphyllobothrium nihonkaiense are humans
C. D. nihonkaiensis can grow up to 4 cm in length
D. D. nihonkaiensis has only salmon as an intermediate host
2. Which one of the following statements regarding the epidemiology of diphyllobothriosis is most accurate?
A. It was most commonly diagnosed in September through November
B. Only a minority of cases were caused by D. nihonkaiensis
C. The timing of cases implicates mature chum salmon as a principal intermediate host
D. Most cases were reported in large metropolitan areas
3. What should you consider regarding the clinical presentation of diphyllobothriosis as you evaluate this patient?
A. Most patients were female
B. Few patients noted the expulsion of strobili while defecating
C. The most common presentation was no clinical symptoms
D. Weight loss occurred in nearly one quarter of patients
4. Which one of the following treatments was used in all cases of diphyllobothriosis in the current study?
A. Praziquantel
B. Mebendazole
C. Metronidazole
D. Erythromycin
Original Publication Date: July 13, 2018
1Retired.
Related Links
Table of Contents – Volume 24, Number 8—August 2018
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Hiroshi Ikuno, BML Inc., Department of Bacteriology, 1361-1 Matoba, Kawagoe City, Saitama 350-1101, Japan
Top