Volume 25, Number 12—December 2019
CME ACTIVITY - Research
Streptococcus suis–Associated Meningitis, Bali, Indonesia, 2014–2017
Introduction

Medscape CME ACTIVITY
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; and (4) view/print certificate.
Release date: November 15, 2019; Expiration date: November 15, 2020
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the epidemiology and clinical signs of Streptococcus suis meningitis, according to a case series in Bali, Indonesia
• Determine laboratory findings and microbiology of S. suis meningitis, according to a case series in Bali, Indonesia
• Identify clinical and public health implications of findings from this case series of S. suis meningitis in Bali, Indonesia
CME Editor
Karen L. Foster, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Karen L. Foster has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Ni Made Susilawathi, MD; Ni Made Adi Tarini, MD; Ni Nengah Dwi Fatmawati, MD, PhD; I Putu Bayu Mayura, MD; Anak Agung Ayu Suryapraba, MD; Made Subrata, DVM; Anak Agung Raka Sudewi, MD, PhD; and Gusti Ngurah Mahardika, DVM, PhD, have disclosed no relevant financial relationships.
Abstract
Streptococcus suis is an emerging agent of zoonotic bacterial meningitis in Asia. We describe the epidemiology of S. suis cases and clinical signs and microbiological findings in persons with meningitis in Bali, Indonesia, using patient data and bacterial cultures of cerebrospinal fluid collected during 2014–2017. We conducted microbiological assays using the fully automatic VITEK 2 COMPACT system. We amplified and sequenced gene fragments of glutamate dehydrogenase and recombination/repair protein and conducted PCR serotyping to confirm some serotypes. Of 71 cases, 44 were confirmed as S. suis; 29 isolates were serotype 2. The average patient age was 48.1 years, and 89% of patients were male. Seventy-seven percent of patients with confirmed cases recovered without complications; 11% recovered with septic shock, 7% with deafness, and 2% with deafness and arthritis. The case-fatality rate was 11%. Awareness of S. suis infection risk must be increased in health promotion activities in Bali.
Community-acquired bacterial meningitis is a serious infectious disease with high rates of illness and death worldwide, even in the era of effective antimicrobial drugs (1). The disease is classified as a neurologic emergency; thus, immediate diagnosis and accurate treatment are vital to save the patient’s life (2). Gram-positive, coccus-shaped Streptococcus suis (3) is the most common causative agent of zoonotic bacterial meningitis; pigs are the primary source of infection. S. suis is an important pathogen in community-acquired bacterial meningitis (2,4,5).
Human S. suis infections are mostly associated with pig husbandry and eating pork-derived products. Since 2010, the number of reported S. suis infections in humans has increased substantially; most cases have originated in Southeast Asia, where the density of pigs is high (6). Moreover, >1,600 S. suis infections have been reported in 30 countries worldwide (7). Previously considered to be sporadic, S. suis meningitis can cause epidemics, as occurred in Thailand, Vietnam, and China (3). The presence of this bacterium is likely to be inevitable in areas with dense pig populations, including the province of Bali in Indonesia. We describe data on the epidemiology, clinical signs, and microbiology of S. suis from meningitis cases in Bali.
Data Collection
We obtained medical records of persons who had suspected bacterial meningitis during 2014–2017 from the Sanglah Provincial Referral Hospital (SPRH; Denpasar, Bali, Indonesia). SPRH is a 760-bed national referral hospital for eastern Indonesia with >600,000 annual visits.
Cerebrospinal fluid (CSF) was collected from each patient at admission. Recorded data included patient demographics and clinical signs indicating bacterial meningitis, such as altered mental status, fever, headache, and neck stiffness (8). Other data were CSF laboratory test results, therapy history, and outcomes.
Laboratory Investigation
We cultured CSF samples from patients with suspected meningitis on a 5% defibrinated sheep blood agar plate (DSBAP) and incubated in 5% CO2 at 37°C for 18–24 h (9). We isolated colonies for identification and drug susceptibility testing using fully automatic VITEK 2 COMPACT system (bioMérieux, https://www.biomerieux.com) based on Clinical and Laboratory Standards Institute guidelines (10). Upon positive detection, we grew selected colonies in tryptic soy broth, incubated at 37°C for 18–24 h, and preserved at –80°C in 50% glycerol. We cultured 44 glycerol stock isolates of S. suis on DSBAP and incubated in 5% CO2 at 37°C for 18–24 h for further study. We reconfirmed the bacterial identity using VITEK 2 COMPACT.
PCR and Sequencing
We suspended 6–8 colonies grown on DSBAP in 200 μL of phosphate buffered saline (pH 7.3) and then isolated bacterial DNA using a Roche High Pure PCR Template Isolation Kit (Roche Life Science, https://www.roche.com). DNA was eluted with 50 μL of elution buffer. We confirmed all isolates by PCR using glutamate dehydrogenase (GDH) and recombination/repair protein (recN) primer sets, as described previously (11,12). We commercially sequenced selected PCR products in 1stBase (Selangor, Malaysia), aligned them using MEGA 6.0 (https://www.megasoftware.net), and subjected them to BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi). We inferred phylogenetic reconstruction of GDH sequence using the unweighted pair group method with arithmetic mean (13). We downloaded the GDH or parts of complete genomes of S. suis from GenBank for reference and included 1 sequence of S. pneumoniae in the phylogenetic analysis. We conducted PCR serotyping to confirm serotype 2 and 1/2, as well as 1 and 14, using published primer sets (14). Further differentiation of serotype 2 to serotype 1/2 was based on BLAST of recN.
Ethics Approval
The Research Ethics Committee of the Faculty of Medicine, Udayana University (Denpasar, Bali, Indonesia), approved this study (no. 691/UN.14.2/KEP/2017, dated April 7, 2017). In accordance with the standard operation procedure of the SPRH, CSF was collected after informed consent.
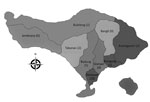
Of 71 acute bacterial meningitis cases, S. suis was confirmed in CSF culture of 44 patients (Table 1). The median time from illness onset to hospital admission was 2 days (range 1–14 days). Thirty-nine (89%) patients were male; the average patient age + SD was 48.1 + 11.5 (range 28–77 years). The most common 3 municipalities/regencies of origin of patients were Denpasar (28 [64%] confirmed cases), Badung (5 [11%]), and Gianyar (4 [9%]) (Figure 1). Patient occupations were private sector employees (57%), unemployed (14%), farmers (11%), entrepreneurs (11%), and government employees (7%).
The 4 most frequent clinical signs in patients with acute S. suis meningitis were fever (91%), neck stiffness (86%), altered mental status (86%), and headache (82%) (Table 2). Septic shock was documented in 5 (11%) cases and sensorineural hearing loss in 4 (9%); seizure, ataxia, and hemiparesis were each recorded in 3 cases (7%) and arthritis in 2 (5%).
All patients were treated intravenously with 2 g of ceftriaxone every 12 hours for 14 days and 10 mg of dexamethasone every 6 hours for 4 days. In 2 patients, meningitis relapsed after 14 days of ceftriaxone treatment, but they recovered after 3 additional weeks of ceftriaxone therapy. The case-fatality rate (CFR) was 11%; moderate disabilities occurred in 16% of survivors in the form of sensorineural deafness (4 patients) and hemiparesis (3 patients).
Complete blood counts showed leukocytosis (mean + SD 24.4 + 10.5 × 103 cells/μL) (Table 3). The neutrophil differential count was 88.4% + 9.8%, and the lymphocyte count was 4.9% + 4.7%. The mean platelet count was 196.4 + 100.2 × 103 cells/μL. CSF analysis showed pleocytosis (median 799 cells/μL; range 92–8,510 cells/μL); CSF neutrophil count was 60%, and lymphocyte count was 40%. Glucose levels were low (median 5 mg/dL; range 0–78 mg/dL); the CSF/blood glucose ratio was 0.4; and protein levels were increased (median 198 mg/dL; range 64–855 mg/dL). CSF culture was positive for S. suis and sensitive to ceftriaxone, benzyl-penicillin, ampicillin, levofloxacin, erythromycin, vancomycin, and linezolid (data not shown).
PCR results for GDH and recN of all samples produced specific single bands of expected sizes (data not shown). Five GDH and 3 recN PCR products were sequenced. The sequences of GDH and recN generated in this study are available in GeneBank (accession nos. MK161045–54). All GDH and recN sequences of S. suis generated in our study were identical. BLAST analysis of the GDH sequence, using blastn (15), demonstrated that the sequence had query cover of 100% and an identity score of 94%–100% with the complete S. suis genome, GDH complete or partial cDNA sequences (CDS). S. pneumoniae and S. marmotae had a 99%–100% query cover and an identity score of 86%. For recN, the sequence from the isolates had query cover of 100% and identity of 95%–99% with the S. suis complete genome and S. suis recN partial CDS. The next closest query cover of 54% with identity score of 83% was with the recN CDS of S. parasuis. Phylogenetic analysis of GDH (Figure 2) showed that the isolates were identical with 20 GDH and part of complete genome sequences of S. suis.
PCR serotyping showed that 29 (66%) of the 44 isolates were positive in PCR using primer pair for serotype 2 or 1/2, which amplifies cps2I gene, whereas none were positive using primer pair for serotypes 1 and 14 detecting cps1I, as previously published (14). The sequence of cps2I of our isolates are available in GenBank (accession nos. MN395406–34). The readable length of sequences was 284 bp. The sequences were identical to S. suis cps2I gene of the reference sequence (GenBank accession no. KC537364) (14). BLAST analysis showed the recN of our isolates were distancing 3.7% to the strain 2651 (GenBank accession no. AB724091), which was annotated as serotype 1/2 (19).
Our study confirms that S. suis is present and infects human in Bali. This finding should alert other provinces in Indonesia. The bacterium has been isolated previously in other provinces (20,21), but the cases of human S. suis meningitis we report extend the known range of S. suis in Indonesia. Pigs are raised in many provinces in Indonesia, and densities differ. In 13 provinces, pig populations were >100,000 head in 2017 (https://www.bps.go.id). The presence of S. suis in other provinces needs to be confirmed. Pigs or pig products are thought to be the main source of human infection (6) because evidence on the role of other species is unavailable. The awareness will be invaluable in avoiding human suffering and death because medical services will be fully informed and aware of the risk posed by S. suis and thus better equipped to save lives.
We based this study on medical records of persons with suspected bacterial meningitis during 2014–2017 at SPRH. All patients with suspected meningitis in the province are referred to this hospital for a definitive diagnosis. Although the presence of S. suis has been confirmed only since 2014, suspected bacterial meningitis had been suspected before then and diagnosed as S. viridans group. The installment of VITEK 2 COMPACT testing confirmed S. suis in 2014. Although cases from many districts in Bali might have been underdiagnosed, we believe that the number of confirmed cases in this report represents most human cases in the province.
Handling pigs or pork products seems to be the major risk factors for human transmission of S. suis (22). Pork products can originate from slaughterhouses, as has been described in Vietnam (23), or from backyard slaughter of dead or sick pigs, as reported in China (24). The risk also seems to increase when raw pork products are eaten. Furthermore, eating raw or medium-cooked pork-derived food containing blood, tonsil, tongue, intestine, and uterus has been indicated as an important risk factor for S. suis meningitis (25,26). A history of ingesting raw pork, pig’s blood, or both was found in most cases in Thailand (27,28).
Eating raw meat with fresh blood from sick or subclinically infected pigs might be a major risk factor for S. suis transmission in humans in Bali, Indonesia. Most (88%) confirmed S. suis meningitis patients in our study were men. This finding was similar to that of S. suis infection in Thailand (28). The average age and the proportion of men is consistent with the results of a systematic review of studies published during 1980–2015 (2). The link of traditional pork consumption and pig handling to the risk for contracting S. suis needs to be elucidated further in Bali.
S. suis was predominant as the causal agent of acute bacterial meningitis in our study. Our finding shows it was confirmed in 44 (62%) of 71 acute bacterial meningitis cases. The percentage might have been higher because the S. suis–negative patients received antimicrobial therapy before sampling. Human infection with this bacterium needs immediate interventions. Recent data from SPRH showed 20 confirmed cases in 2018 and 13 as of July 2019.
Clinical signs of S. suis meningitis recorded in this study resemble those of general bacterial meningitis (2,4,6,29). All cases were of acute infection. The median time from illness onset to hospital admission in our study was 2 days (range 1–14 days). The 4 most frequent clinical signs were fever or history of fever, neck stiffness, altered mental status, and headache; these signs correspond to the 3 most frequent globally reported symptoms of meningitis: fever, headache, and neck stiffness (2,30).
Initially, some patients did not demonstrate overt neurologic symptoms and thus were admitted under nonneurologic diagnoses. One patient was admitted to the Ear, Nose and Throat Department for sensorineural bilateral deafness, and another was admitted as having an ischemic stroke. Another patient was admitted with suspected dengue fever, which later developed into clinical meningitis. Such misadmission is understandable and may be more widespread because infection with S. suis has been reported to cause other syndromes, such as arthritis, endocarditis, peritonitis, and endophthalmitis (29–31).
If we grade outcomes according to the Glasgow Outcome Scale (32), 73% of the patients in our study had favorable outcomes. All 44 patients were intravenously treated for bacterial meningitis with 2 g of ceftriaxone every 12 hours for 14 days and 10 mg of dexamethasone every 6 hours for 4 days, in accordance with SPRH protocol. Ceftriaxone is a third-generation cephalosporin, which is recommended as the drug of choice for bacterial meningitis (6,8).
The CFR in our study was 11%; death was caused by septic shock, which has been attributed to S. suis infection (2,33). The CFR here is slightly higher than the globally reported CFR of ≈3% (2). The reported CFR for S. suis meningitis is lower than for other bacterial meningitis, such as pneumococcal (20%) and Listeria monocytogenes (36%) meningitis (2). The relatively high CFR seems to be related to the late admission of some patients in our study. A high CFR has also been reported in Thailand (34).
Four (9%) patients reported hearing loss in our study. This percentage is lower than that from previous findings. In a systematic review and meta-analysis to summarize global estimates of the epidemiology, clinical characteristics, and outcomes of S. suis infection, hearing loss was reported in ≈40%–50% of cases and vestibular dysfunction in >20% (2,29). This discrepancy might be due to early administration of antimicrobial drugss, so S. suis was uncultivable. Also, we excluded unconfirmed cases from our study.
Laboratory findings in the CSF were leukocytosis (predominantly neutrophil), low glucose levels, and increased protein content. These findings resemble typical bacterial meningitis (1,8).
Of all S suis serotypes, serotype 2 is recognized as the most common pig and human pathogen (23,35). However, other serotypes should not be ignored, as evidenced by serotype 5 in Japan (36), serotype 9 in Thailand (37), serotype 16 in Vietnam (38), serotype 21 in Argentina (39), serotypes 24 (40) and 31 (41) in Thailand, and many more. PCR serotyping indicated that 29 of 44 isolates were positive in PCR using a primer set to detect serotypes 2 and 1/2 (14) but not serotypes 1 and 14. We focused on serotypes 2 and 1/2 because S. suis serotype 2 is the most common cause of human cases (42); serotypes 1, 4, 14, and 16 infection can lead to severe illness, but fewer cases are reported than for serotype 2 (38). We confirmed those PCR-positive isolates in our study to be serotype 2 or 1/2. The readable sequences were identical to S. suis cps2I gene of the reference sequence (GenBank accession no. KC537364) (14). Although the existing PCR serotyping is unable to differentiate between serotype 2 and 1/2 (14), the nucleotide sequences of recN of our isolates are distancing 3.7% to the 2651 strain (GenBank accession no. AB724091), which was annotated as serotype 1/2 (19). Therefore, we proposed those PCR-positive isolates were serotype 2. Samples should be sent to a reference laboratory to be tested using a panel of standard antiserum (6), and the complete primer sets for PCR serotyping (14) serotypes of all isolates should be made available. The knowledge gained will convey important epidemiologic picture for human prevention.
We confirmed S. suis in this study after applying a standard method with fully automatic equipment. Performing PCR and sequencing of GDH and recN further confirmed the species identification. Both gene fragments are proposed as an appropriate PCR system for the reclassification of S. suis (11) or as a specific PCR system for S. suis (12).
BLAST search of the GDH sequences showed high coverage and identity with the S. suis complete genome and GDH partial CDS available in the database. The closest identity score of 86% was to S. pneumoniae and S. marmotae. Phylogenetic analysis (Figure 2) also confirmed that our isolates are S. suis. The recN had high sequence coverage and high identity to the S. suis database, too., The closest sequence data of S. parasuis have an identity score of 83% to the recN of S. suis. Sequencing of PCR products to confirm detected genetic sequences should limit or reduce misidentification. We did not sequence all PCR products because sequencing was conducted only to determine the specificity of the PCR. We propose implementation of GDH and recN as diagnostic tools in elucidating the distribution of S. suis in Indonesia.
Misidentification of S. suis is common. This bacterium is frequently misidentified as S. viridans (43) and has also been misidentified as S. bovis, S. pneumoniae, S. faecalis, and S. acidominimus (29,44). Misidentification of S. suis also has been reported in Canada, which raises suspicion that human S. suis infections might be underdiagnosed in North America (45). We found 1 case of suspected S. mitis infection using the VITEK 2 COMPACT system. However, PCR and sequencing confirmed this to be S. suis.
Published reports of animal cases and isolation of S. suis from animals in Bali are not available. Isolation of S. suis from tonsil samples has been reported from Papua, Indonesia (21). Another group in Udayana University is working to isolate and detect S. suis from sick pigs in Bali, further suggesting that S. suis is present in the island (K. Besung, Udayana University, pers. comm., 2018 Oct 1). As indicated elsewhere that pig and pork products are the primary sources of human infection (2,4,5), so is the source of S. suis in humans in our study most likely to be pigs and pork products.
In conclusion, we confirmed S. suis meningitis in humans in Bali, Indonesia. Of 44 cases, 29 human isolates were serotype 2. Because human infections are mostly associated with pig husbandry and eating pork-derived products, the distribution of S. suis in the country needs to be fully elucidated. The risk factor of eating raw pork and pig blood in traditional delicacies seems to be valid, although this point requires further investigation. Our study contributes to enhancing knowledge of S. suis distribution and risk factors in Bali. By increasing awareness of S. suis infection, medical services will be better prepared to alleviate human suffering and death from S. suis meningitis.
Dr. Susilawathi is a lecturer at the Faculty of Medicine, Udayana University, and is undertaking a doctoral degree in the School of Post Graduate Studies of Udayana University. Her primary research interests include neurologic infection and pathogenesis.
Acknowledgments
We thank Gillian Campbell for editing a draft of this manuscript.
This study was funded by a Research Group Grant of The Research and Development of the Faculty of Medicine, Udayana University of Bali, in 2017.
References
- van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074. DOIPubMedGoogle Scholar
- van Samkar A, Brouwer MC, Schultsz C, van der Ende A, van de Beek D. Streptococcus suis meningitis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9:
e0004191 . DOIPubMedGoogle Scholar - Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, et al. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence. 2014;5:477–97. DOIPubMedGoogle Scholar
- van Samkar A, Brouwer MC, van der Ende A, van de Beek D. Zoonotic bacterial meningitis in human adults. Neurology. 2016;87:1171–9. DOIPubMedGoogle Scholar
- Gottschalk M, Fittipaldi N, Segura M. Streptococcus suis meningitis. In: Christodoulides M, editor. Meningitis: cellular and molecular basis. Oxford (UK): CAB International; 2013. p. 184–98.
- Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48:617–25. DOIPubMedGoogle Scholar
- Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3:
e45 . DOIPubMedGoogle Scholar - Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008;7:637–48. DOIPubMedGoogle Scholar
- Lehman DC, Mahon CR, Suvarna K. Streptococcus, Enterococcus, and other catalase-negative, gram-positive cocci. In: Mahon CR, Lehman DC, Manuselis G, editors. Textbook of diagnostic microbiology. 5th ed. Maryland Heights (MO): Saunders Elsivier; 2016. p. 328–48.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement (M100-S27). Wayne (PA): The Institute; 2017. p. 250.
- Ishida S, Tien HT, Osawa R, Tohya M, Nomoto R, Kawamura Y, et al. Development of an appropriate PCR system for the reclassification of Streptococcus suis. J Microbiol Methods. 2014;107:66–70. DOIPubMedGoogle Scholar
- Okwumabua O, O’Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett. 2003;218:79–84. DOIPubMedGoogle Scholar
- Sneath PHA, Sokal RR. Numerical taxonomy. San Francisco: Freeman; 1973.
- Liu Z, Zheng H, Gottschalk M, Bai X, Lan R, Ji S, et al. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One. 2013;8:
e72070 . DOIPubMedGoogle Scholar - Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. DOIPubMedGoogle Scholar
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. DOIPubMedGoogle Scholar
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. DOIPubMedGoogle Scholar
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. DOIPubMedGoogle Scholar
- Tien HT, Nishibori T, Nishitani Y, Nomoto R, Osawa R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA-DNA homology and sodA and recN phylogenies. Vet Microbiol. 2013;162:842–9. DOIPubMedGoogle Scholar
- Southeast Asia Infectious Disease Clinical Research Network. Causes and outcomes of sepsis in southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Health. 2017;5:e157–67. DOIPubMedGoogle Scholar
- Nugroho W, Cargill CF, Putra IM, Kirkwood RN, Trott DJ, Salasia SI, et al. Investigations of selected pathogens among village pigs in Central Papua, Indonesia. Trop Anim Health Prod. 2016;48:29–36. DOIPubMedGoogle Scholar
- Zalas-Wiecek P, Michalska A, Grabczewska E, Olczak A, Pawlowska M, Gospodarek E. Human meningitis caused by Streptococcus suis. J Med Microbiol. 2013;62:483–5. DOIPubMedGoogle Scholar
- Ngo TH, Tran TB, Tran TT, Nguyen VD, Campbell J, Pham HA, et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One. 2011;6:
e17943 . DOIPubMedGoogle Scholar - Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, et al.; Streptococcus suis study groups. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–20. DOIPubMedGoogle Scholar
- Nghia HD, Tu TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. Erratum in: PLoS One. 2011;6(4); 2012;7(5). PLoS One. 2011;6:e17604.
- Takeuchi D, Kerdsin A, Akeda Y, Chiranairadul P, Loetthong P, Tanburawong N, et al. Impact of food safety campaign on Streptococcus suis infection in humans in Thailand. Am J Trop Med Hyg. 2017;96:1370–7. DOIPubMedGoogle Scholar
- Navacharoen N, Chantharochavong V, Hanprasertpong C, Kangsanarak J, Lekagul S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol. 2009;123:857–62. DOIPubMedGoogle Scholar
- Takeuchi D, Kerdsin A, Pienpringam A, Loetthong P, Samerchea S, Luangsuk P, et al. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One. 2012;7:
e31265 . DOIPubMedGoogle Scholar - Huong VT, Ha N, Huy NT, Horby P, Nghia HD, Thiem VD, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20:1105–14. DOIPubMedGoogle Scholar
- Teekakirikul P, Wiwanitkit V. Streptococcus suis infection: overview of case reports in Thailand. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 2):178–83.PubMedGoogle Scholar
- Heidt MC, Mohamed W, Hain T, Vogt PR, Chakraborty T, Domann E. Human infective endocarditis caused by Streptococcus suis serotype 2. J Clin Microbiol. 2005;43:4898–901. DOIPubMedGoogle Scholar
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–59. DOIPubMedGoogle Scholar
- Fillo S, Mancini F, Anselmo A, Fortunato A, Rezza G, Lista F, et al. Draft genome sequence of Streptococcus suis strain SsRC-1, a human isolate from a fatal case of toxic shock syndrome. Genome Announc. 2018;6:e00447–18. DOIPubMedGoogle Scholar
- Wangsomboonsiri W, Luksananun T, Saksornchai S, Ketwong K, Sungkanuparph S. Streptococcus suis infection and risk factors for mortality. J Infect. 2008;57:392–6. DOIPubMedGoogle Scholar
- Robertson ID, Blackmore DK. Experimental studies on the comparative infectivity and pathogenicity of Streptococcus suis type 2. I. Porcine and human isolates in pigs. Epidemiol Infect. 1990;105:469–78. DOIPubMedGoogle Scholar
- Taniyama D, Sakurai M, Sakai T, Kikuchi T, Takahashi T. Human case of bacteremia due to Streptococcus suis serotype 5 in Japan: The first report and literature review. IDCases. 2016;6:36–8. DOIPubMedGoogle Scholar
- Kerdsin A, Hatrongjit R, Gottschalk M, Takeuchi D, Hamada S, Akeda Y, et al. Emergence of Streptococcus suis serotype 9 infection in humans. J Microbiol Immunol Infect. 2017;50:545–6. DOIPubMedGoogle Scholar
- Nghia HD, Hoa NT, Linh D, Campbell J, Diep TS, Chau NV, et al. Human case of Streptococcus suis serotype 16 infection. Emerg Infect Dis. 2008;14:155–7. DOIPubMedGoogle Scholar
- Callejo R, Prieto M, Salamone F, Auger JP, Goyette-Desjardins G, Gottschalk M. Atypical Streptococcus suis in man, Argentina, 2013. Emerg Infect Dis. 2014;20:500–2. DOIPubMedGoogle Scholar
- Kerdsin A, Gottschalk M, Hatrongjit R, Hamada S, Akeda Y, Oishi K. Fatal septic meningitis in child caused by Streptococcus suis serotype 24. Emerg Infect Dis. 2016;22:1519–20. DOIPubMedGoogle Scholar
- Hatrongjit R, Kerdsin A, Gottschalk M, Takeuchi D, Hamada S, Oishi K, et al. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand. BMC Infect Dis. 2015;15:392. DOIPubMedGoogle Scholar
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–9. DOIPubMedGoogle Scholar
- Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health. 2009;40:511–7.PubMedGoogle Scholar
- Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000-2011. Diagn Microbiol Infect Dis. 2012;74:75–7. DOIPubMedGoogle Scholar
- Gomez-Torres J, Nimir A, Cluett J, Aggarwal A, Elsayed S, Soares D, et al. Human case of Streptococcus suis disease, Ontario, Canada. Emerg Infect Dis. 2017;23:2107–9. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on http://www.medscape.org. If you are not registered on http://www.medscape.org, please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@medscape.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to https://www.ama-assn.org. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Article Title:
Streptococcus suis–Associated Meningitis, Bali, Indonesia, 2014–2017
CME Questions
1. Your patient is a 50-year-old male pig farmer in Indonesia admitted for suspected bacterial meningitis. According to the case series in Bali, Indonesia, by Susilawathi and colleagues, which of the following statements about the epidemiology and clinical signs of Streptococcus suis meningitis is correct?
A. Of a total of 71 acute bacterial meningitis cases, S. suis was confirmed in cerebrospinal fluid (CSF) culture of 14 patients.
B. Case-fatality rate in confirmed cases was 5%
C. All patients received 2 g intravenous (IV) ceftriaxone every 12 hours for 14 days and 10 mg IV dexamethasone every 6 hours for 4 days
D. Sensorineural hearing loss was the most common presenting sign
2. According to the case series in Bali, Indonesia, by Susilawathi and colleagues, which of the following statements about laboratory findings and microbiology of S. suis meningitis is correct?
A. CSF cultures were positive for S. suis, sensitive to ceftriaxone, and resistant to all other drugs tested
B. PCR serotyping showed that most cases were S. suis, serotype 1
C. CSF analysis showed pleocytosis with normal glucose levels
D. All glutamate dehydrogenase and recombination/repair protein sequences of S. suis generated in this study were identical
3. According to the case series of S. suis meningitis in Bali, Indonesia by Susilawathi and colleagues, which of the following statements about clinical and public health implications of the findings would be correct?
A. Human S. suis infections are mostly linked to contact with goats and eating goat’s milk or meat
B. The study was likely to overestimate the percentage of bacterial meningitis cases caused by S. suis
C. The presence of S. suis in Indonesia was first confirmed in 2014, but suspected bacterial meningitis was reported earlier and diagnosed as viridans streptococci
D. The specific signs and symptoms of S. suis infection facilitate diagnosis of this type of bacterial meningitis
Original Publication Date: November 15, 2019
Related Links
Table of Contents – Volume 25, Number 12—December 2019
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Gusti N. Mahardika, Udayana University, Animal Biomedical and Molecular Biology Laboratory, Faculty of Veterinary Medicine, Jl Sesetan-Markisa #6, Denpasar, 80226, Bali, Indonesia
Top