Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2–Specific IgG
Sina Schwarzkopf, Adalbert Krawczyk, Dietmar Knop, Hannes Klump, Andreas Heinold, Falko M. Heinemann, Laura Thümmler, Christian Temme, Marianne Breyer, Oliver Witzke, Ulf Dittmer, Veronika Lenz, Peter A. Horn, and Monika Lindemann
Author affiliations: Institute for Transfusion Medicine, University Hospital Essen, University of Duisburg-Essen, Essen, Germany (S. Schwarzkopf, D. Knop, H. Klump, A. Heinold, F.M. Heinemann, L. Thümmler, C. Temme, M. Breyer, V. Lenz, P.A. Horn, M. Lindemann); Department of Infectious Diseases, West German Centre of Infectious Diseases, Universitätsmedizin Essen, University of Duisburg-Essen, Essen (A. Krawczyk, O. Witzke); Institute for Virology, University Hospital Essen, University of Duisburg-Essen, Essen (U. Dittmer)
Main Article
Figure 3
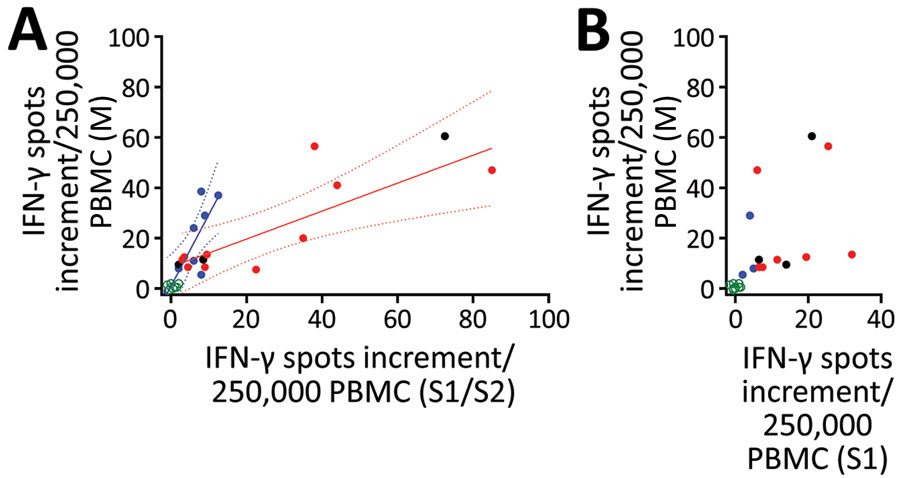
Figure 3. Interrelationship between results of various severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific cellular assays in 78 potential convalescent-plasma donors with PCR-confirmed infection, Germany. The plots include the first dataset in potential convalescent plasma donors and in negative controls. Red dots represent volunteers with an antibody ratio >3; black dots, volunteers with a ratio of 1.1–3; blue dots, volunteers with ratio <1.1; green dots, NC. PBMCs of volunteers were stimulated by peptide pools of the S1/S2 and the M protein and by an S1 protein antigen of SARS-CoV-2. A) Analysis of ELISpot assay with S1/S2 peptides versus M peptides. We performed 2 linear regression analyses separately for potential plasma donors with an IgG ratio >3 and <1.1. Solid lines show regression lines and dotted lines 95% CI. B) Analysis of ELISpot assay with S1 protein versus M peptides. ELISpot, enzyme-linked immunospot; IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cells.
Main Article
Page created: October 15, 2020
Page updated: December 21, 2020
Page reviewed: December 21, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
