Volume 28, Number 6—June 2022
Dispatch
Fasciolopsis buski Detected in Humans in Bihar and Pigs in Assam, India
Abstract
The foodborne intestinal trematode Fasciolopsis buski causes the neglected zoonotic disease fasciolopsiasis. We detected F. buski infection in 14 pediatric patients in Sitamarhi, Bihar, and in pigs in Sivasagar, Assam, India. Proper diagnostic methods and surveillance are urgently needed to accurately estimate the true burden of this disease in India.
Fasciolopsis buski is a foodborne intestinal trematode that causes the neglected zoonotic disease fasciolopsiasis in humans and pigs. F. buski infection is transmitted through ingestion of raw aquatic plants or water carrying encysted metacercaria. Persons with substantial worm loads can have clinical indicators, such as malnutrition, edema, malabsorption, severe diarrhea, ascites, and anemia, and might experience acute intestinal obstruction and ileus (1–3). F. buski worms are found mostly in Asia and the Indian subcontinent; endemicity is highest in eastern India (4). We previously reported multiple cases of infection with Artyfechinostomum sufrartyfex, an echinostome trematode, which was diagnosed in children at Shri Shubh Lal (SSL) Hospital and Research Centre in Sitamarhi, Bihar state, India (5). We also documented several cases of fasciolopsiasis among SSL patients during 2012–2021 and infection in pigs detected in Sivasagar district, Assam state, India during a 2019–2020 survey.
Infections with this parasite have been reported from diverse regions of India, as well as other parts of Asia (6–10). A genetic study suggested that the species found in India is different from species found in China and Vietnam (11). To corroborate the genetic distinctions between the strains found in India and those from China and Vietnam, we determined the complete nuclear ribosomal ITS2 and partial mitochondrial cytochrome c oxidase subunit 1 gene (cox1) sequences of F. buski from the samples recovered from Bihar and Assam and compared them to sequences from isolates from other regions of India, China, and Vietnam. The institutional ethics committee of Sikkim University in Gangtok, India, approved this work (SU/REG/F-1/03/2018/VOL-1/59).
During 2012–2021, a total of 14 children 3–12 years of age were brought for treatment to SSL Hospital for reported loose bowel movements, including watery feces and feces tinged with blood and mucus for >15 days, as well as vomiting, flatulence, abdominal discomfort, pain in the abdomen, fever, loss of appetite, weakness, and passage of flat reddish worms, called paterwa or lal keera in local languages. Eight patients were male and 6 female. Among the male patients, 3 were ≤5, 3 were 6–10, and 2 were >10 years of age; among the female patients, 3 were ≤5 and 3 were ≥10 years of age. All of the patients were of low socioeconomic status and resided near ponds or deep-water rice paddies contaminated with human and animal excreta and snail-infested areas. The patients were habituated to consume raw snails, contaminated water, chestnuts, and vegetables irrigated with contaminated water from nearby ditches.
On physical examination, all patients were pale and malnourished. General and systemic examination revealed persistent diarrhea, dehydration, and vomiting in most and anemia in all of the case-patients. Laboratory investigation revealed most of the patients had eosinophilia, and grade II malnutrition was associated with most patients (Table 1). All patients tested negative on tuberculin and HIV tests, and results from routine urine examination, complete blood counts, serum urea, serum creatinine, serum bilirubin, and alanine aminotransferase testing were within reference ranges (Appendix 1).
Naked eye examination of the feces revealed the presence of live parasites in 2 patients and dead parasites in 12. Two patients had mixed infection: 1 with both F. buski and A. sufrartyfex parasites and the other with F. buski worms and the roundworm Ascaris lumbricoides. On microscopic examination of feces samples, no eggs or ova of intestinal flukes were identified, except 1 sample showed fertilized roundworm ova. We isolated the recovered parasites and processed them for morphologic, anatomic, and genetic analysis. All of the patients were hospitalized and treated with praziquantel (75 mg/kg bodyweight; 3 divided doses for 2 d) and supportive measures administered for dehydration, electrolyte imbalance, and malnutrition. All patients were cured and discharged after being counseled for nutritional rehabilitation.
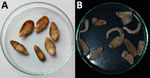
In 2019–2020, a survey of pigs for F. buski infection was performed in the Charaideo, Sivasagar, Lakhimpur, Biswanath, and Tezpur districts of Assam. A total of 128 pigs were examined; 3 in the Sivasagar district displayed evidence of parasite infection. The flukes were collected in 0.9% phosphate-buffered saline (pH 7.2) from the intestines of freshly slaughtered pigs in Sivasagar district, as well as from the feces and vomitus of SSL Hospital patients, and brought to the Sikkim University Department of Zoology for further analysis (Figure 1).
We extracted and purified genomic DNA from the flukes collected from both patients and pigs using QIAGEN DNeasy Blood and Tissue Kit (https://www.qiagen.com) according to manufacturer instructions. We performed amplification and sequencing of the complete ITS2 and partial cox1 genes using the primers 3S: 5′-GGTACCGGTGGATCACTCGGCTCGTG-3′ (forward), A28: 5′-GGGATCCTGGTTAGTTTCTTTTCCTCCGC-3′ (reverse) (12,13), and DICE 1F: 5′-TTWCNTTRGATCATAAG, Dice 14R: 5′-CCHACMRTAAACATATGATG-3′ (reverse) (14). The ITS2 amplicon was ≈292 bp and the cox1, ≈784 bp. We uploaded sequences to GenBank (ITS2 accession nos. MW771525 [Sitamarhi] and MW771526 [Sivasagar]; cox1 accession nos. MW767135 [Sitamarhi] and MW767136 [Sivasagar]. We identified parasites using a BLASTn search (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
The ITS2 sequences of the Sitamarhi and Sivasagar isolates were genetically similar and showed the greatest sequence similarity with previously identified F. buski isolates from Uttar Pradesh (GenBank accession no. KF564866) and Meghalaya, India (accession no. DQ351841), with little or no genetic variability. In contrast, F. buski sequences from China and Vietnam (GenBank accession nos. MN970005 and EF612489) had 7.7%–8.2% genetic difference from the isolates from India. However, the sequences from China and Vietnam were identical to each other. Similarly, the cox1 sequences from Sitamarhi and Sivasagar exhibited only 0.4% variation between each other but 12.1%–12.3% variation from sequences from Vietnam (accession no. MF287794) and China (accession no. KX169163). The sequences from China and Vietnam had only 0.5% variation from each other (Table 2; Appendix 2 Figures 1, 2).
We constructed phylogenetic trees based on the 2 gene regions using the maximum-likelihood method. Both trees clearly showed the Indian isolates forming a separate clade from the isolates from China and Vietnam (Figure 2). On the basis of our findings, we concluded that the samples collected in our study belonged to F. buski but that the isolates from China and Vietnam were separate taxa from those from India; however, F. buski samples from China and Vietnam were the same species. This discovery is consistent with the findings of a prior study in China (11).
Our study confirmed that the parasites obtained from both human patients in Sitamarhi and pigs in Sivasagar were of the F. buski species. However, we also corroborated that the species found in India might differ from those in China and Vietnam, and species taxonomy might need to be revised in the future (11).
In recent years, F. buski infection from humans and pigs has been documented in India in the states of Assam, Bihar, Delhi, Meghalaya, and Uttar Pradesh. According to our findings, this parasite is an increasing public health threat in India, especially in remote locations and among persons from low socioeconomic backgrounds, because of the substantial risk to human and animal health it poses. Surveys are urgently needed to determine the true burden of fasciolopsiasis in the country. A lack of effective diagnostic tools for detecting neglected foodborne trematode infections, including F. buski, means there are no prevalence data on these infections in the country. Therefore, there is a pressing need to design and develop a rapid and easy detection tool for F. buski and other neglected trematode infections.
Ms. Saikia is a PhD student in the Department of Zoology, School of Life Sciences, Sikkim University in Gangtok. Her scientific interests include helminth parasitology, particularly using the proteomics of foodborne trematodes to identify novel therapeutic targets and diagnostic markers. Dr. Prasad was director, consultant pediatrician, and neonatologist at Shri Shubh Lal Hospital and Research Centre, Sitamarhi, Bihar, India. His primary research interest was pediatric parasitology.
Acknowledgments
We thank CSIR and UGC, the Government of India, New Delhi, for Research Fellowship (UGC-JRF) to DS and the hospital staff at Shri Shubh Lal Hospital and Research Centre in Sitamarhi for providing help with sample collection.
We dedicate this work to the loving memory of our beloved senior colleague and coauthor of this paper, the recently deceased Dr. Yugal Kishore Prasad.
References
- Achra A, Prakash P, Shankar R. Fasciolopsiasis: endemic focus of a neglected parasitic disease in Bihar. Indian J Med Microbiol. 2015;33:364–8.
- Fried B, Graczyk TK, Tamang L. Food-borne intestinal trematodiases in humans. Parasitol Res. 2004;93:159–70. DOIPubMedGoogle Scholar
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–78. DOIPubMedGoogle Scholar
- Ranjan S, Saurabh K, Prasad RR. Gastrointestinal manifestations of Fasciolopsis buski associated polyparasitism in patients of an endemic area: a hospital based study. Int J Community Med Public Health. 2017;4:1898–900. DOIGoogle Scholar
- Prasad YK, Dahal S, Saikia B, Bordoloi B, Tandon V, Ghatani S. Artyfechinostomum sufrartyfex trematode infections in children, Bihar, India. Emerg Infect Dis. 2019;25:1571–3.
- Prasad PK, Tandon V, Chatterjee A, Bandyopadhyay S. PCR-based determination of internal transcribed spacer (ITS) regions of ribosomal DNA of giant intestinal fluke, Fasciolopsis buski (Lankester, 1857) Looss, 1899. Parasitol Res. 2007;101:1581–7. DOIPubMedGoogle Scholar
- Jha AK, Jha SK. Endoscopic diagnosis of Fasciolopsis buski: revisited (with video). JGH Open. 2019;4:284–6.
- Mahajan RK, Duggal S, Biswas NK, Duggal N, Hans C. A finding of live Fasciolopsis buski in an ileostomy opening. J Infect Dev Ctries. 2010;4:401–3. DOIPubMedGoogle Scholar
- Swargiary A, Roy B, Giri BR, Ronghang B. A comparative study on the anthelmintic efficacy of some medicinal plants of North-East India: alteration in the glycolytic enzymes of Fasciolopsis buski, a giant intestinal fluke. Asian Pac J Trop Med. 2013;6:412–20.
- Singh UC, Kumar A, Srivastava A, Patel B, Shukla VK, Gupta SK. Small bowel stricture and perforation: an unusual presentation of Fasciolopsis buski. [PubMed]. Trop Gastroenterol. 2011;32:320–2.PubMedGoogle Scholar
- Ma J, Sun MM, He JJ, Liu GH, Ai L, Chen MX, et al. Fasciolopsis buski (Digenea: Fasciolidae) from China and India may represent distinct taxa based on mitochondrial and nuclear ribosomal DNA sequences. Parasit Vectors. 2017;10:101. DOIPubMedGoogle Scholar
- Bowles J, Blair D, McManus DP. A molecular phylogeny of the human schistosomes. Mol Phylogenet Evol. 1995;4:103–9. DOIPubMedGoogle Scholar
- Blair D, Agatsuma T, Watanobe T, Okamoto M, Ito A. Geographical genetic structure within the human lung fluke, Paragonimus westermani, detected from DNA sequences. Parasitology. 1997;115:411–7. DOIPubMedGoogle Scholar
- Moszczynska A, Locke SA, McLaughlin JD, Marcogliese DJ, Crease TJ. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol Ecol Resour. 2009;9(Suppl s1):75–82. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: May 13, 2022
1These first authors contributed equally to this article.
Table of Contents – Volume 28, Number 6—June 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sudeep Ghatani, Assistant Professor, Department of Zoology, School of Life Sciences, Sikkim University, 5th Mile, Tadong, Gangtok 737102, Sikkim, India
Top