Volume 29, Number 1—January 2023
Dispatch
Successful Treatment of Balamuthia mandrillaris Granulomatous Amebic Encephalitis with Nitroxoline
Abstract
A patient in California, USA, with rare and usually fatal Balamuthia mandrillaris granulomatous amebic encephalitis survived after receiving treatment with a regimen that included the repurposed drug nitroxoline. Nitroxoline, which is a quinolone typically used to treat urinary tract infections, was identified in a screen for drugs with amebicidal activity against Balamuthia.
Balamuthia mandrillaris is an ameba that can cause skin lesions, endophthalmitis, or granulomatous amebic encephalitis (GAE) in previously healthy patients. Routes of infection include possible soil exposure (1) and transplanted organs from infected donors (2,3). The mortality rate is high. During 1974–2016, of 109 Balamuthia GAE cases in the United States, 10 patients survived; mortality rate was >90% (4).
The recommended medication regimen for Balamuthia GAE includes pentamidine, sulfadiazine, azithromycin/clarithromycin, fluconazole, flucytosine, and miltefosine (5). This regimen is based on survivor case series, and results have been inconsistent. Of those medications, only miltefosine, pentamidine, and azithromycin exhibit in vitro evidence of amebicidal or amebistatic activity (6,7). Furthermore, in vitro activity requires high concentrations of drug that are implausible in vivo (50% inhibitory concentration for azithromycin 244 μM and miltefosine 62 μM) (6). We report a patient with B. mandrillaris granulomatous amebic encephalitis who survived after receiving treatment with nitroxoline, a drug typically used to treat urinary tract infections, which was identified in a screen for drugs with amebicidal activity against Balamuthia.
The patient was a man in his 50s, with no remarkable medical history, who received care at a hospital in northern California, USA, after experiencing a generalized seizure. Magnetic resonance imaging (MRI) demonstrated a solitary left temporal lobe T2 hyperintensity with gadolinium rim enhancement and surrounding edema. After receiving treatment with dexamethasone and levetiracetam, he was transferred to the University of California San Francisco Medical Center (UCSF) (Figure 1).
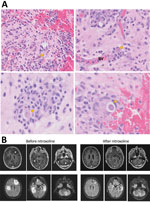
Examination by neurology consultants indicated disorientation, inattention, moderate aphasia, and mild right hemiparesis. The aphasia and hemiparesis improved during hospitalization, suggesting that those signs were postictal. Cerebrospinal fluid (CSF) testing revealed increased nucleated cells up to 80/UL (60% lymphocytes, 17% neutrophils, 23% monocytes), protein concentration 38 mg/dL, and glucose concentration 100 mg/dL. A biopsy sample from the left temporal lobe lesion was preliminarily reported as well-formed granulomata with acute inflammation (Figure 2, panel A).
Cultures from the brain biopsy sample did not grow bacteria, fungi, or mycobacteria. We performed metagenomic next-generation sequencing (mNGS) on a CSF sample (8,9) and sent brain biopsy samples for universal broad-range PCR amplicon sequencing (uPCR) for bacteria, fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Rereview of neuropathology raised concern for amebic forms, prompting us to add uPCR of brain biopsy sample for ameba species (10). The patient was discharged.
On day 26 after the initial visit, Sanger sequencing of the uPCR amplicon was positive for B. mandrillaris. This diagnosis was confirmed by research-based mNGS of DNA extracted from the formalin-fixed, paraffin-embedded brain tissue under a remnant clinical sample biobanking protocol (UCSF IRB #10-01116; Appendix) (11). Results of all other studies, including mNGS of CSF, were negative.
On day 27, the patient was rehospitalized, again with disorientation and inattention. Repeat MRI showed disease progression with multiple new supratentorial and infratentorial lesions consistent with amebic abscesses. After consulting with the Centers for Disease Control and Prevention, we started administering sulfadiazine (1,500 mg orally 4×/d), fluconazole (1,000 mg [12 mg/kg] orally 1×/d), flucytosine (3,000 mg [37.5 mg/kg] 4×/d), pentamidine (330 mg [4 mg/kg] intravenously 1×/d), azithromycin (500 mg orally 1×/d), miltefosine (50 mg orally 3×/d), and albendazole (400 mg orally 1×/d) (Figure 1).
MRI on day 42 (15 days of the 7-drug regimen) showed moderately reduced lesion size, reduced edema, and no new lesions. However, severe medication toxicities developed. Hypoglycemia required discontinuation of pentamidine. Subsequent renal failure caused by sloughing of renal calyces required discontinuation of sulfadiazine and placement of bilateral nephrostomy tubes. The patient’s kidney function improved but remained impaired (baseline estimated glomerular filtration rate 20). Decreased absolute neutrophil count necessitated stopping and then reducing flucytosine dose. The ongoing limited regimen included dose-reduced flucytosine, fluconazole, miltefosine, albendazole, and azithromycin. Brain MRI on day 59 (14 days on this limited regimen) showed interval increase in lesion size, with increased edema.
Given his worsening prognosis, the patient and his family agreed to trial treatment with nitroxoline, a quinolone antibiotic with in vitro amebicidal activity against B. mandrillaris (6). Nitroxoline was selected instead of compounds identified in other high-throughput screens (12) because it is available internationally to treat urinary tract infections and is well tolerated (13). Nitroxoline was authorized through an emergency use authorization (Food and Drug Administration Investigational New Drug no. 154939), and on day 103, we initiated treatment (250 mg orally 3×/d). All other medications were continued. The patient experienced a transient acute kidney injury; although the nephrology staff considered it unrelated to nitroxoline, we submitted a possible adverse event report.
On day 109, after 1 week of nitroxoline treatment, MRI showed decreased size of the cerebral abscesses and no new lesions compared with MRI before nitroxoline on day 96 (Figure 2, panel B). The patient was discharged, although neurologic examination continued to demonstrate mild disorientation and inattention. Recovery was complicated by a subsequent acute kidney injury because of malfunctioning ureteral stents, and nitroxoline administration was stopped while creatinine clearance was <20 (days 122–143). Interval MRIs on days 156 and 220 (7 and 17 weeks after nitroxoline initiation) showed continued marked improvement in lesion size (Figure 2, panel B).
As of 15 months after initial evaluation, the patient continues to take nitroxoline, miltefosine, azithromycin, albendazole, fluconazole, and dose-reduced flucytosine. His infectious disease outpatient clinicians plan to sequentially discontinue medications after 1 year. He lives in the community, and his family assists with medication management and appointments.
Repurposed use of nitroxoline associated with survival from B. mandrillaris GAE demonstrates the potential of basic research to identify antiamebic agents that improve outcome of this rare and deadly disease. Given the limited options for treating Balamuthia GAE, several studies have used high-throughput screening tools to identify other amebicidal agents. Compounds from the Medicines for Malaria Ventures Pandemic Response Box underwent in vitro trials against B. mandrillaris, but the leading candidates have not been used in humans or are not commercially available (12). In the screening for the patient reported here, which identified nitroxoline as having amebicidal activity against B. mandrillaris, in vitro efficacy of nitroxoline against B. mandrillaris cysts and trophozoites was determined to be higher than that of currently recommended drugs and prevented tissue destruction in fibroblast and explant models of B. mandrillaris infection (6).
In vitro efficacy of nitroxoline is better than that of other antiamebic medications; it also is safe and well tolerated. In a review of its use in urinary tract infections, 9.8% of patients reported adverse events, primarily nausea (13). In contrast, current antiamebic treatments can cause severe toxicity, especially pentamidine and sulfadiazine. It is not known if nitroxoline penetrates the CNS, but our limited experience suggests that it may, especially in a patient with a compromised blood–brain barrier.
A barrier to treating Balamuthia GAE is diagnostic delay. Imaging characteristics are nonspecific, and more common pathologies are often misdiagnosed (e.g., neoplasm or pyogenic abscesses) (4). CSF findings are typically nonspecific, direct detection tests of CSF can be insensitive, and visualization of organisms in CSF is rare. Thus, diagnosis often requires brain biopsy, specialized pathology, or dedicated PCR testing.
Unbiased diagnostics, such as mNGS, offer the opportunity to streamline and accelerate diagnosis of rare pathogens such as Balamuthia (8). Multiple Balamuthia GAE cases have been diagnosed by CSF mNGS (14,15). In the case we report, results of CSF mNGS testing were negative, concordant with negative CSF uPCR and indicating that no abscesses had ruptured or were abutting the ventricles (8). However, results of uPCR and research-based mNGS of brain tissue were positive. Currently, clinical mNGS is not available for brain biopsy samples; clinical validation of this test may speed diagnoses of future cases. Despite the limitations of any case study, we suggest that accelerated diagnosis with unbiased techniques and early initiation of nitroxoline may offer promise to improve survival rates for patients with Balamuthia GAE.
Dr. Spottiswoode is a research fellow in the Division of HIV, Infectious Diseases, and Global Medicine at the University of California San Francisco. Her research interests include the use of novel diagnostic tools and the combination of pathogen metagenomics and host responses to understand disease pathophysiology and outcome.
Acknowledgments
We thank the patient and his family for participating in this study.
The Free-Living and Intestinal Ameba Laboratory, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, provided advice and guidance. Asieris Pharmaceuticals provided the nitroxoline pro bono. Heather Stone guided emergency authorization. David Sears, Dong Lee, Malcolm John, and Scott Grapentine were instrumental in preparing the Emergency Use Authorization and caring for the patient. Michael Reid and Erika Wallender provided advice. University of Washington Molecular Microbiology provided the full sequence of the universal PCR. Written informed consent was obtained from the patient for publication of this manuscript.
J.D.R. received funding from the Chan-Zuckerberg Biohub. M.R.W. received funding from the Westridge Foundation.
References
- Jackson BR, Kucerova Z, Roy SL, Aguirre G, Weiss J, Sriram R, et al. Serologic survey for exposure following fatal Balamuthia mandrillaris infection. Parasitol Res. 2014;113:1305–11. DOIPubMedGoogle Scholar
- Farnon EC, Kokko KE, Budge PJ, Mbaeyi C, Lutterloh EC, Qvarnstrom Y, et al.; Balamuthia Transplant Investigation Teams. Balamuthia Transplant Investigation Teams. Transmission of Balamuthia mandrillaris by organ transplantation. Clin Infect Dis. 2016;63:878–88. DOIPubMedGoogle Scholar
- Gupte AA, Hocevar SN, Lea AS, Kulkarni RD, Schain DC, Casey MJ, et al. Transmission of Balamuthia mandrillaris through solid organ transplantation: utility of organ recipient serology to guide clinical management. Am J Transplant. 2014;14:1417–24. DOIPubMedGoogle Scholar
- Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, Moser M, et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974-2016. Clin Infect Dis. 2019;68:1815–22. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Parasites—Balamuthia mandrillis—granulomatous amoebic encephalitis (GAE) [cited 2022 Jan 31]. https://www.cdc.gov/parasites/balamuthia/treatment.html
- Laurie MT, White CV, Retallack H, Wu W, Moser MS, Sakanari JA, et al. Functional assessment of 2,177 U.S. and international drugs identifies the quinoline nitroxoline as a potent amoebicidal agent against the pathogen Balamuthia mandrillaris. MBio. 2018;9:e02051–18. DOIPubMedGoogle Scholar
- Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003;41:3175–80. DOIPubMedGoogle Scholar
- Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380:2327–40. DOIPubMedGoogle Scholar
- Miller S, Naccache SN, Samayoa E, Messacar K, Arevalo S, Federman S, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29:831–42. DOIPubMedGoogle Scholar
- Rakeman JL, Bui U, Lafe K, Chen YC, Honeycutt RJ, Cookson BT. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J Clin Microbiol. 2005;43:3324–33. DOIPubMedGoogle Scholar
- Ramachandran PS, Ramesh A, Creswell FV, Wapniarski A, Narendra R, Quinn CM, et al. Integrating central nervous system metagenomics and host response for diagnosis of tuberculosis meningitis and its mimics. Nat Commun. 2022;13:1675. DOIPubMedGoogle Scholar
- Rice CA, Lares-Jiménez LF, Lares-Villa F, Kyle DE. In vitro screening of the open-source Medicines for Malaria Venture Malaria and pathogen boxes to discover novel compounds with activity against Balamuthia mandrillaris. Antimicrob Agents Chemother. 2020;64:e02233–19. DOIPubMedGoogle Scholar
- Naber KG, Niggemann H, Stein G, Stein G. Review of the literature and individual patients’ data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect Dis. 2014;14:628. DOIPubMedGoogle Scholar
- Haston JC, Rostad CA, Jerris RC, Milla SS, McCracken C, Pratt C, et al. Prospective cohort study of next-generation sequencing as a diagnostic modality for unexplained encephalitis in children. J Pediatric Infect Dis Soc. 2020;9:326–33. DOIPubMedGoogle Scholar
- Wilson MR, Shanbhag NM, Reid MJ, Singhal NS, Gelfand JM, Sample HA, et al. Diagnosing Balamuthia mandrillaris encephalitis with metagenomic deep sequencing. Ann Neurol. 2015;78:722–30. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: December 19, 2022
1These authors contributed equally to this article.
Table of Contents – Volume 29, Number 1—January 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Natasha Spottiswoode, Division of HIV, Infectious Disease, and Global Medicine, University of San Francisco, 513 Parnassus Ave, S380, San Francisco, CA 94143, USA
Top