Volume 30, Number 3—March 2024
Research Letter
Enterocytozoon bieneusi Infection after Hematopoietic Stem Cell Transplant in Child, Argentina
Abstract
We report a case of Enterocytozoon bieneusi infection in a pediatric hematopoietic stem cell transplant recipient in Argentina. Spores were visualized in feces using Calcofluor White and modified trichrome stainings. PCR and sequencing identified E. bieneusi genotype D in fecal samples and liver samples, confirming extraintestinal dissemination of the parasite.
Microsporidia, fungal-related single-cell parasites, infect a broad range of vertebrates and invertebrates. The most identified species of Microsporidia in humans are Enterocytozoon bieneusi and Encephalitozoon intestinalis, which have emerged as opportunistic pathogens in immunosuppressed persons, such as those infected with HIV, organ transplant recipients, and cancer patients. The infective forms of these parasites are the resistant spores that persist in the environment, causing infections through direct contact with infected persons, infected animals, or ingestion of contaminated water and food (1). Human microsporidiosis is characterized primarily by chronic diarrhea and wasting, with less frequent occurrences of extraintestinal disseminated disease. Identification to the genus and species level is crucial for tailored treatments, especially in cases of chronic diarrhea (2).
Pediatric patients undergoing allogeneic hematologic stem cell transplantation (HSCT) may experience gut-localized or extraintestinal microsporidiosis by Encephalitozoon spp (3). In patients with leukemia or lymphoma who receive cytotoxic treatments, intestinal infections are predominantly associated with E. bieneusi, and rare cases of extraintestinal dissemination also have been reported (1,4).
More than 500 worldwide genotypes of E. bieneusi have been identified based on genetic polymorphisms in the internal transcribed spacer of the rRNA gene. They are distributed into 11 distinct phylogenetic groups, with groups 1 and 2 comprising genotypes with zoonotic potential that infect humans and various mammalian and avian species (2).
Although intestinal microsporidiosis is prevalent in children residing in developing countries, scarce studies have been reported in Argentina (1,5,6). We present a case of E. bieneusi (genotype D) infection in a child who underwent unrelated allogeneic HSCT in Buenos Aires, Argentina.
A 12-year-old boy from Buenos Aires who had a January 2018 diagnosis of intermediate-risk pre-B acute lymphoblastic leukemia received an unrelated allogeneic HSCT in February 2022. A month after HSCT, the child was treated with antiviral therapy for reactivation of cytomegalovirus, adenovirus, and Epstein-Barr virus infections. Three months post-HSCT, under immunosuppressive therapy with tacrolimus (0.1 mg/kg/d), he received antimicrobial treatment with meropenem (60 mg/kg/d), linezolid (30 mg/kg/d), and liposomal amphotericin B (3 mg/kg/d) to combat prolonged fever and abdominal symptoms. Videoendoscopy of the upper digestive tract confirmed gastrointestinal graft-versus-host disease, and ultrasound showed splenomegaly with multiple rounded hypodense images in the spleen and liver. We also noted distension of the ileal and colonic loops, predominantly in the right colon, and ascites.
We treated the child with liposomal amphotericin B (3 mg/kg/d) to address persistent febrile symptoms and visceral lesions compatible with chronic disseminated candidiasis. Four months after HSCT, the child sought treatment for chronic diarrhea (>1 month) and abdominal pain. Prior to microbiological documentation, we prescribed empirical treatment of metronidazole (30 mg/kg/d), which produced no improvement of symptoms.
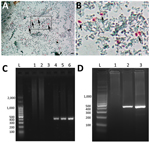
Coproanalysis revealed typical polymicrobial bacterial flora, with no detection of bacterial toxins, adenovirus, rotavirus, or parasites. Calcofluor White and Weber’s modified trichrome staining revealed structures compatible with microsporidian spores in single and serial fecal specimens (Figure, panels A, B). Analysis of liver aspiration biopsy samples rendered no conclusive results. On the basis of microscopic results, we immediately initiated albendazole treatment (400 mg/d) for microsporidiosis (7).
We conducted molecular biology studies based on fecal samples and liver aspiration biopsy samples. We determined E. bieneusi and genotype identification by using a nested PCR protocol that targeted the entire internal transcribed spacer and also amplified portions of the flanking large and small subunits of the ribosomal RNA (≈400 bp) gene (8,9) (Figure, panels C, D). We confirmed the presence of E. bieneusi genotype D based on Sanger sequencing using the inner-nested PCR primers (2). We named the nucleotide sequence generated BsAs1 and deposited it into GenBank (accession no. OP650902). Despite a decrease in diarrhea symptoms, the child died 18 days after initiation of albendazole treatment due to fulminant hyperacute lymphoproliferative syndrome, before identification of E. bieneusi was determined.
E. bieneusi has been reported commonly in cancer patients undergoing chemotherapy (1,3,4,10). We report a case of E. bieneusi genotype D microsporidiosis, with intestinal and hepatic localization, in a child with leukemia and immunosuppression after a bone marrow transplant in Argentina. Our findings highlight the need to incorporate microsporidiosis in the differential diagnosis of immunosuppressed children after transplant surgery, as well as for other patient populations at high risk for opportunistic infections. Our report also emphasizes the critical importance of microsporidia identification because albendazole is effective against some Encephalitozoon species but not against E. bieneusi (1,7). Genotyping isolates of clinical E. bieneusi may help to identify potential environmental sources. Although nitazoxanide could be used as an alternative treatment, fumagillin has a wider range of activity effectively targeting E. bieneusi (7). The unavailability of fumagillin for treating human infections in several countries, including Argentina, underscores the need for enhanced accessibility to microsporidia treatment options, especially for vulnerable populations.
Bioq. Mena is a biochemist and PhD student at the National University of Cordoba and fellow of CONICET, Córdoba, Argentina. His research interests include development and improvement of molecular tools for diagnosis and epidemiology of parasite and fungal human pathogens causing infections in South America. Bioq. Pérez Garófalo is biochemist at the Laboratorio Central, Hospital de Pediatría S.A.M.I.C. “Prof. Dr. Juan P. Garrahan,” Buenos Aires, Argentina. Her research interests include improvement of diagnosis techniques for infectious diseases in pediatric patients.
Acknowledgment
This study was approved by the Research and Ethics Committee of Hospital de Pediatría J.P. Garrahan (Buenos Aires, Argentina). This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Fondo para la Investigación Científica y Tecnológica (FONCyT), Argentina, PICT 2019-4101 and SECyT-UNC. C.J.M is a Fellow from CONICET. L.S.C. is Researcher of CONICET.
References
- Han B, Pan G, Weiss LM. Microsporidiosis in Humans. Clin Microbiol Rev. 2021;34:
e0001020 . DOIPubMedGoogle Scholar - Li W, Feng Y, Santin M. Host specificity of Enterocytozoon bieneusi and public health implications. trends Parasitol. 2019;35:436–51.
- Ambrosioni J, van Delden C, Krause KH, Bouchuiguir-Wafa C, Nagy M, Passweg J, et al. Invasive microsporidiosis in allogeneic haematopoietic SCT recipients. Bone Marrow Transplant. 2010;45:1249–51. DOIPubMedGoogle Scholar
- Jiménez-González GB, Martínez-Gordillo MN, Caballero-Salazar S, Peralta-Abarca GE, Cárdenas-Cardoz R, Arzate-Barbosa P, et al. [Microsporidia in pediatric patients with leukemia or limphoma]. Rev Invest Clin. 2012;64:25–31.PubMedGoogle Scholar
- Velásquez JN, di Risio C, Etchart C, Chertcoff AV, Astudillo OG, Carnevale S. Multimethodological approach to gastrointestinal microsporidiosis in HIV-infected patients. Acta Parasitol. 2019;64:658–69. DOIPubMedGoogle Scholar
- Valperga SM, de Jogna Prat SA, de Valperga GJ, Lazarte SG, de Trejo AV, Díaz N, et al. [Microsporidian spores in the stool specimens of toddlers, with or without diarrhea, from Tucumán, Argentina]. Rev Argent Microbiol. 1999;31:157–64.PubMedGoogle Scholar
- Han B, Weiss LM. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin Ther Targets. 2018;22:903–15. DOIPubMedGoogle Scholar
- Buckholt MA, Lee JH, Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol. 2002;68:2595–9. DOIPubMedGoogle Scholar
- Mena CJ, Barnes A, Castro G, Guasconi L, Burstein VL, Beccacece I, et al. Microscopic and PCR-based detection of microsporidia spores in human stool samples. Rev Argent Microbiol. 2021;53:124–8. DOIPubMedGoogle Scholar
- Desoubeaux G, Nourrisson C, Moniot M, De Kyvon MA, Bonnin V, De La Bretonniére ME, et al. Genotyping approach for potential common source of Enterocytozoon bieneusi infection in Hematology unit. Emerg Infect Dis. 2019;25:1625–31. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: February 08, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Laura S. Chiapello, Departamento de Bioquímica Clínica (Lab 105), Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, CIBICI, CONICET, Ciudad Universitaria, Haya de la Torre y Medina Allende, X5000HUA Córdoba, Argentina
Top