Volume 9, Number 3—March 2003
Synopsis
Electron Microscopy for Rapid Diagnosis of Emerging Infectious Agents1
Abstract
Diagnostic electron microscopy has two advantages over enzyme-linked immunosorbent assay and nucleic acid amplification tests. After a simple and fast negative stain preparation, the undirected, “open view” of electron microscopy allows rapid morphologic identification and differential diagnosis of different agents contained in the specimen. Details for efficient sample collection, preparation, and particle enrichment are given. Applications of diagnostic electron microscopy in clinically or epidemiologically critical situations as well as in bioterrorist events are discussed. Electron microscopy can be applied to many body samples and can also hasten routine cell culture diagnosis. To exploit the potential of diagnostic electron microscopy fully, it should be quality controlled, applied as a frontline method, and be coordinated and run in parallel with other diagnostic techniques.
In late September 2001, a letter containing spores of Bacillus anthracis arrived at a publishing house in Palm Beach, Florida, and resulted in the death of one employee from inhalation anthrax. Over the next 6 weeks, similar letters were delivered to television news centers in New York City and government offices in Washington, D.C. Ultimately >32,000 suspected exposures and five deaths were recorded in the United States. The collateral spread of exposure to spores was a sobering reminder of the bioterrorism attack scenario hypothesized by O’Toole (1).
Today, technology allows genetic engineering of potentially devastating agents such as modified ectromelia virus (2), the weaponizing variola virus (former USSR) (3), the long distance dispersal of yellow fever–infested mosquitos (United States) (4), and the weaponizing of anthrax spores by many nations. The ease with which the recent anthrax attacks were delivered indicates that unsophisticated methods are still effective. Thus, the most potent defenses remain rapid identification of the event and agent, treatment of the victims, and containment of infection. Successful outbreak management depends on early recognition of a suspected infectious case by the primary care physician and obtaining an accurate, timely laboratory diagnosis. An unexpected temporal or geographic cluster of illness of apparently infectious nature or an unusual age distribution of pneumonia with respiratory failure, intradermal hemorrhage, or chickenpox-like illnesses may indicate infection caused by a novel agent or a bioterrorist act. Similarly, the sudden appearance of vesicular lesions or respiratory illness in farm animals may be evidence of an emerging disease, a possible zoonosis, or an agriterrorist act. While recent studies suggest that health-care systems are ill prepared to treat victims and contain the spread of an infectious agent (5), the performance of physicians, epidemiologists, and diagnostic specialists in identifying outbreak-associated agents as diverse as Nipah virus (6) and gastroenteric agents (7) indicate that identification of an outbreak and its associated agent may be done rapidly and successfully.
Electron microscopic diagnosis is uniquely suited for rapid identification of infectious agents. A specimen can be ready for examination and an experienced virologist or technologist can identify, by electron microscopy, a viral pathogen morphologically within 10 minutes (8). We describe the role of transmission electron microscopy in viral diagnosis and outbreak management; methods for specimen collection, preparation, and examination; laboratory safety and quality control; and the differential morphologic diagnosis of infectious agents. In addition, an Appendix lists support facilities that provide electron microscopic diagnostic service.
The first electron micrograph of poxvirus was published in 1938. In 1941, immunologic procedures were first used in electron microscopic studies of tobacco mosaic virus (9), and electron microscopy was introduced successfully in the differential diagnosis of smallpox and chickenpox infections in the late 1940s (10,11). With the introduction of negative staining in the late 1950s (12) and the wider availability of electron microscopes, electron microscopy (as a catchall method) became essential in characterizing many new isolates detected in diagnostic cell cultures and clinical samples, e.g., stool, urine, and biopsied specimens (7,13–16). Pattern recognition, i.e., information on size and particle morphology, leads to rapid identification of infectious agents. The initial classification of many agents was therefore based on a combination of morphology and genome structure. Currently, >30,000 different viruses comprising 56 separate families have been identified, and humans have been found to host 21 of the 26 families specific for vertebrates (17). The distinct morphology of members of different viral families usually allows an agent to be assigned to a particular family. This morpho-diagnosis, combined with clinical information is, in most cases, sufficient to permit a provisional diagnosis or rule out a more serious infection and to initiate treatment and containment protocols without waiting for other test results.
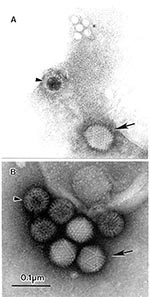
Because electron microscopy is not suitable for screening large numbers of samples, many alternate immunologic and molecular methods have been developed on the basis of nucleic acid amplification techniques. While immunologic tests have almost unlimited throughput, the high specificity of these assays may result in failure to identify etiologic agents with different antigenic determinants. Further, reagents may not currently exist that would permit complete immunologic testing (18,19). Even when an immunologic test is appropriate for the etiologic agent, the sensitivity may only equal that of electron microscopy (20,21). Nucleic acid amplification techniques have similar limitations. They are more sensitive but are only capable of identifying the presence of genomic material for previously identified agents. Although primers exist that will permit amplification of most enteroviruses (22,23), few multiplex systems can identify all genotypes and serotypes within, or between, the different families of viruses that infect humans (22,24,25). Further, mutations in the primer target region may negate the effectiveness of primers. Because nucleic acid amplification techniques will not identify subviral components such as empty virions, which may be produced late in an infection, some studies suggest that their practical level of sensitivity does not always exceed that of electron microscopy (19,25,26). Because this modern armament has taken over most routine diagnostics, with the exception of gastroenteric viral infections, electron microscopy may be concentrated on infectious disease emergencies. The “open view” of electron microscopic testing allows an unbiased, rapid detection of viruses and other agents if sufficiently high particle concentrations exist (Figure 1). Because of this capability, electron microscopic testing must be a frontline method, applied either to samples directly from a suspected lesion, bodily fluids, or biopsies after cell-culture augmentation of a cultivable agent or from letters and environmental samples.
Successful investigation of any outbreak or novel case starts with specimen collection. Insufficient, improper, or inadequate sampling may delay or prevent identification of a causative agent. Sufficient sampling requires identification of, and sampling from, all areas where infection may have been established. Fecal samples are ideal for investigating gastroenteric episodes, as are lesion fluids or smears from skin lesions of possible viral origin.
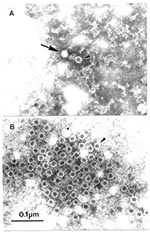
A major cause of insufficient sampling can be failure to collect acute-phase sera from affected case-patients and their contacts. First, the existence of a blood-borne pathogen may not be evident when examining unexplained cases, as demonstrated by the difficulty identifying HIV (27) and hepatitis C virus infections, and associating human parvovirus B-19 with Fifth disease (16). Second, acute-phase sera are essential for demonstrating seroconversion to a suspected agent. Third, clinical symptoms may be caused by an immune response to an infection that has resolved by the time they appear. However, specimens from apparently uninfected contacts of patients with acute cases may contain the agent involved (16). Convalescent-phase sera collected from case-patients 4–6 weeks after onset of illness are also powerful diagnostic reagents. If no agent has been identified by standard virus detection procedures (e.g., electron microscopy, tissue culture, immunoassay, or nucleic acid amplification techniques), these serum samples may be used to detect the causative agent (28), while matched acute:convalescent-phase serum pairs collected at least 2 weeks apart may be used to demonstrate a significant rise in specific antibody among cases by immuno-electron microscopy (Figure 2) (7). Infectious agents may also be identified in cerebrospinal fluid, lesion crusts, nasopharyngeal washes, saliva, tears, urine, and biopsied tissue specimens (29). However, low viral load, sampling difficulties, or both may reduce the effectiveness of rapid electron microscopic diagnosis on these later types of specimens without initial tissue culture amplification, as observed in Nipah virus studies (6).
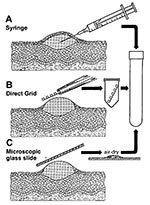
Safety concerns, miscommunication between infectious diseases specialists and staff who collect samples, or inadequate training may result in improper sample collection. Although swab samples placed into viral transport media may allow nucleic acid amplification techniques or culture of nonfastidious agents to be carried out; such specimens are not very conducive to successful rapid electron microscopic diagnosis of lesion exudates caused by dilution effects and interfering components. Several effective ways of collecting lesion fluids exist (8). A method readily available to the physician or in a hospital ward is collection into the barrel of a 26-gauge needle attached to a tuberculin syringe. A fresh lesion is unroofed or the beveled surface of the needle is placed against the base of an open lesion, and fluid is aspirated into the barrel. After capping, the sample may be transported directly for rapid electron microscope diagnosis (Figure 3A). Alternatively, coated electron microscopic specimen grids may be lightly touched directly to the vesicle fluid, lesion base, or both; allowed to air dry; and transported directly for examination (direct touch preparation) (Figure 3B). Because repreparing the sample with direct touch preparations may not be possible, at least two grids should be obtained when the specimen is collected. For safety and containment of hazardous infectious materials, the syringe or grid should be placed in a rigid sterile container, e.g., conical 15-mL centrifuge tube or Beem capsule (Beem Co., Bronx, NY), sealed with Parafilm (American National Can Co., Greenwich, CT), and the outside of the tube washed with 0.5% sodium hypochlorite (10% household bleach) before transport (Figure 3). Safety regulations usually require further packaging of the sample inside a second container.
In the late 1940s, direct touch preparations from skin lesions were prepared in North Africa and sent to Toronto, Canada, where they were examined successfully for smallpox virus for up to 4 months after collection (11). In another comparative study in Winnipeg, Canada, which used matched lesions, we observed an average increase of 10.2:1 in the number of virions visualized by direct touch as opposed to needle aspirate preparations, and the ratio was >1.0 in 92% of total cases examined (n=12; p<0.02; [Wilcoxon signed-rank test]). We observed no difference in the number of positive identifications or homogeneity of virion distribution on the grid between these two methods. Lesion smears on glass slides may also be used effectively for both electron microscopy and immunofluorescent microscopy examination (Figure 3C). Smears, i.e., dried down vesicle fluids, are especially effective when syringes and electron microscopic grids are not available. Both direct touch and smear preparations are useful when specimens must be transported some distance for electron microscopic examination.
Results from lesion exudates, collected as swab samples and placed in viral transport medium, are less useful. A change in specimen collection protocols in 1995, from direct touch/lesion aspirates to swab specimens in transport medium, has resulted in a decline in successful identification of virions in lesion specimens in Winnipeg from 62% to 75% to approximately 10% (Hazelton, unpub. data). While complete fecal samples are preferable, collecting rectal swab samples for diagnosis of gastroenteric agents may be necessary. These swab samples should be placed in capped conical centrifuge tubes with 0.2 mL sterile, distilled water, sealed with Parafilm, and sent for electron microscopic diagnosis. Lesion crusts should also be placed in sterile conical tubes. The addition of any liquid medium to lesion crusts, cerebrospinal fluid, nasopharyngeal washes, saliva, tears, and urine will not assist the electron microscope laboratory. Tissue biopsy samples in buffer without fixatives should be stored at 4°C and sent directly to both an electron microscope facility and a viral identification laboratory for rapid electron microscopy and other diagnostic testing. Fixation may interfere with antibody binding and thus preclude infectivity tests and successful application of any immunoelectron microscopy.
Finally, failure to collect an adequate volume of sample will limit the tests that may be used and the ability to successfully identify causative agents. Lesion fluids are deceiving. For example, samples containing poxvirus or varicella zoster virus that appear to have no material drawn into a needle barrel (Figure 3A) or attached to a grid may still contain numerous virions. When possible, at least 1 g of fecal material should be collected into a commercial stool collection vessel. A minimum of 5.0 mL of blood should be collected into tubes without anticoagulants. When a special interest in the case or outbreak occurs, large samples may provide reagents for later testing. All samples should be immediately sent for rapid electron microscopic diagnosis, with storage at 4°C if possible. Dried smears may be stored and transported at ambient temperature. Under no condition should samples be frozen for storage and transport before receipt at the diagnostic facility (30).
Protecting staff and containing infectious agents are important considerations in the handling of all clinical specimens. Samples may contain agents that are highly infectious or associated with a high mortality rate. In consideration of the possibility of bioterrorism and agri-terrorism, delivering samples to a central facility at biological safety level (BSL) 3 or higher may be necessary for inactivation before electron microscopic examination. Regardless, preparation must be done in a laminar flow hood with BSL-2 or greater containment capability. Most infectious agents may be inactivated in suspension by adding formaldehyde or glutaraldehyde (20 min, final concentration 2% and 0.5%, respectively). Alternatively, hazardous samples may be inactivated after they are mounted on the grid by treating the grid with fixative, by subjecting stained preparations to ultraviolet irradiation (UV) for 5 min before removing them from the biological safety cabinet, or both. UV treatment may, however, affect both virion morphology and grid stability. Prolonged treatment with glutaraldehyde or formaldehyde has little effect on morphology while inactivating most agents (31). Both formaldehyde and glutaraldehyde immobilize structures by Schiff reactions involving aldehyde side groups. As a di-aldehyde with a 5-carbon backbone, glutaraldehyde is more effective than formaldehyde at intermolecular crosslinking. Glutaraldehyde may, therefore, cause aggregation and obscure some fine structural detail. Samples suspected of containing spores should be inactivated with 10% formaldehyde final concentration because spores are more resistant to chemical inactivation (32). Specimens that may contain prions require more harsh treatment, such as the addition of 1 M NaOH, to inactivate the samples. However, treatment with NaOH will degrade most biologic structures to an indecipherable tangle of artefacts, and is, therefore, not conducive to electron microscopic examination.
Specimens that have not been inactivated must still be treated as potentially infectious after electron microscopic examination. For example, no decrease was observed in a 50% tissue culture infective dose (TCID50) of poliovirus samples after they were mounted on the grid and stained with 2.5 mM (1.6%) phosphotungstic acid, pH 7.0. Subsequent exposure to vacuum and the electron beam for 1 min reduced TCID50 by at least 106.5 and 107.5 for adenoviruses and polioviruses, respectively. More importantly, 10-min vacuum and electron beam exposure of grids containing sporulating B. subtilis preparations permitted colony recovery in 60% of tests and reduced colony-forming units 500-fold, and exposure to either vacuum or phosphotungstic acid-negative stain alone had little effect on the viability of adenovirus, poliovirus, or spore preparations (33). These observations underline the extreme resistance of spores in different weapons delivery systems. Because of the risk for residual infectivity, all grids must be disposed of as infectious waste, and equipment used to handle samples and grids, e.g., forceps, must be decontaminated by treatment with 5% glutaraldehyde for 20 min. Alternatively, equipment may be disinfected with 1 M NaOH. Cleaning is also necessary to prevent false-positive results caused by crossover contamination between specimens. Staff involved in rapid electron microscopy should be vaccinated for multiple agents, including smallpox and hepatitis B.
While rapid electron microscopy may be performed with any type of specimen, the requirement for truly rapid electron microscopic diagnosis is not common. Indicators include limiting exposure in clinically threatening situations in which an infectious cause is not ruled out, as may occur if a patient has suspected herpetic lesions in a ward for immunocompromised, newborn, or transplant patients; new clinical symptoms are observed with immunocompromised patients; the need to initiate early treatment; or the risk of passing infection during birth. Since a viral agent may be found by rapid electron microscopy in over 90% of poxvirus (34) and other skin lesions of viral etiology (Gelderblom and Hazelton, unpub. data), this method is ideal for investigating outbreaks of rash-like illness and suspected cases of bioterrorism.
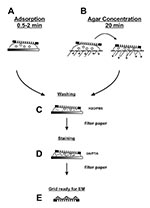
A morphologic diagnosis may be obtained within 10 min of specimen arrival in the electron microscopic facility. The standard two-step drop method, i.e., adsorption followed by negative staining, is used for preparation (Figure 4A). Viral load is usually more than sufficient to allow successful diagnosis of herpesvirus, poxvirus, and some gastroenteric infections. Negative-stain examination is simple and may be conducted in any electron microscopic facility. The first item needed is a 400-mesh electron microscopic grid coated with either a single plastic layer or a plastic film reinforced with carbon (32,35,36). Carbon-coated plastic films have higher thermal stability and are less prone to specimen movement during examination. However, they may be more hydrophobic than plain plastic films. Electron microscope units that specialize in virus preparative or diagnostic techniques prepare their own plastic-coated, carbon-stabilized films, and glow discharge the films to improve hydrophilicity, particle adherence, and distribution of both sample and stain (36,37). Coated grids may also be purchased through most electron microscopy suppliers. Clinical samples with high concentrations of protein often do not require glow discharge pretreatment to reduce hydrophobicity.
Lesion fluids received in the barrel of a needle or capillary tube are expelled onto a hydrophobic surface such as Parafilm. If the sample has dried, a small drop of redistilled water (15 µL),
sterilized through a 0.2-µm–pore filter, is drawn into the specimen container and washed back out. If required, an aliquot of suspension should immediately be transferred to viral transport medium and submitted for cell culture, nucleic acid amplification techniques, and other virologic procedures. Lesion crusts and biopsy material may be soaked in 3 volumes buffer and solubilized by 10–12 pestle strokes in a Dounce homogenizer, while fecal material may be suspended by vortexing with glass beads in 3–9 volumes of distilled water. Heavy debris is allowed to settle, and the suspension cleared by low-speed centrifugation (1,000 x g for 5 min). Liquid samples (cerebrospinal fluid, nasopharyngeal washes, saliva, tears, and urine) may be used directly. If required, an equal volume of double concentration fixative may be mixed with the suspension to inactivate any infectious agents present before mounting the sample on the coated grid. A grid is floated with the coated surface on a drop of fixed suspension for 0.5–2 min and excess material wicked away with an edge of filter paper (Figure 4C). If bacteria are to be negatively stained, higher numbers of microorganisms will attach to the grid because of sedimentation when the drop is placed on the grid. Adsorption is not an absolute process. Any extra manipulations, such as washing the grid, may reduce the number of adsorbed particles. Pretreating the carbon-reinforced grids by glow discharge, poly-L-lysine, alcian blue, or UV light may also help for tighter binding (32,35) and are particularly useful when staining aldehyde-inactivated samples. Direct touch lesion fluid preparations, which are already mounted on the grid, may be rehydrated and inactivated before staining by floating the grid on a drop of fresh 2% formaldehyde.
Rapid immunologic methods that improve sensitivity when searching for unknown agents include solid-phase immunoelectron microscopic (SPIEM) (38) and serum in agar (SIA) (39), both of which may use either pooled human immunoglobulins (HuIgG) or specific antibodies. HuIgG may be obtained from most immunologic suppliers or hospital pharmacies. SPIEM concentrates antigens on the grid by immune capture, thereby improving the probability of observing an etiologic agent. The coated surface of a grid is floated on a drop of pooled HuIgG (100 µg/mL and 20 µg/mL in phosphoate-buffered saline [PBS] B) or antiserum (1/100 and 1/500 in PBS) for 10 min, washed on 6 sequential drops PBS, and floated on the specimen for 30–60 min at 37°C. The sample may be stabilized after SPIEM with 0.1% glutaraldehyde to ensure tight binding of the captured antigens, washed on 6 drops of PBS, negative stained, and examined (38). SIA uses immunoaggregation to identify antigens. In addition, type-specific antisera may be used in SIA to serotype the agent present. Antibody (1/100 for antisera and 100 µg/mL for HuIgG) is prepared in cooled 1% agar. A grid is placed on the solidified agar, and a drop of sample placed over the grid. Diluent diffuses into the agar while antibody diffuses into the suspension and antigen:antibody complexes form, which then adsorb to the grid as diluent volume is reduced (Figure 4B) (39).
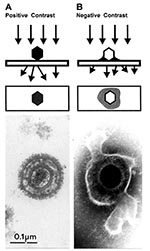
Biologic structures, because of low mass density, interact weakly with electrons used for imaging, and therefore, show little contrast or detail. Several ways exist to generate sufficient image contrast and resolution; the most versatile is positive and negative staining with heavy metal ions, e.g., lead, tungsten, and uranium ions. Positive staining depends on chemical reactivity with the components of the object and involves fixation, postfixation, embedding in resins, ultrathin sectioning, and multiple staining incubations. These procedures may take 4–5 days before a sample is ready for examination. Rapid embedding protocols can reduce the time to approximately 1 day but with a loss in specimen quality (32). In contrast, negative staining is simple, rapid, and well suited for examination of small particulate suspensions. A coated grid with sample adsorbed to the surface is floated on a drop of negative stain for 0.5–2 min, excess stain wicked away with a piece of filter paper, air dried for 1–3 min, and examined by electron microscopy (Figure 4D). Structures on the grid are surrounded and stabilized by the drying stain. Thus, they appear as transparent, highly detailed negative images within a dark halo of stain (Figure 5B).
The most common negative stains are 1% (60 mM) aqueous uranyl acetate, pH 2-4.5, and 1% (2.5 mM) phosphotungstic acid, pH adjusted to 7.0 with NaOH. Aqueous uranyl acetate is unstable at higher pH values. Because aqueous uranyl acetate and phosphotungstic acid differ in staining properties, both stains should be applied in parallel in case of unknown samples. Stains should be relatively fresh and stored in brown glass bottles at 4°C (32,35,36). While examining the stained grid, additional grids may be left floating on the sample droplet, protected from dust and drying. This method reduces preparation time in the event additional grids must be prepared for electron microscopic inspection.
If no virus has been identified after 20 min or after the examination of 10 grid squares, the result may be considered to be no etiologic agent identified. Routine two-step drop preparations for electron microscopic diagnostic procedures require particle concentrations of 106 to 108/mL. Therefore, negative evidence is not an absolute diagnosis. A number of effective concentration or immunologic procedures exist that markedly increase sensitivity of electron microscopic diagnostics for samples with lower particle concentrations (32,40). These procedures take from 0.5 to 16 hours and are labor and training intensive. Viral research or diagnostic facilities generally have access to at least one advanced procedure. Nonimmunologic procedures include: a) ultracentrifuge concentration—the material from cleared suspensions is sedimented by ultracentrifugation, resuspended in a smaller volume and then prepared by the standard two-step drop method (32); b) agar diffusion—a 20–50 [20- to 50-µL drop of suspension is placed on 1% agar. As the fluid is absorbed the virus is concentrated. After 15–20 min, a grid is placed on the remaining suspension and then stained as with the two-step method above (Figure 4D). This procedure will result in an enrichment factor of approximately 5x (32); and c) direct centrifugation to the electron microscopic grid with the Beckman Airfuge (Beckman, Palo Alto, CA) EM-90 rotor or A-100 rotor, a procedure that increases sensitivity up to 1,000 fold (40–42). Immunoaggregation and immunodecoration with type- and genus-specific antibody may be used to concentrate material or to specifically identify the agent, e.g., herpes simplex 1 and 2 and varicella zoster. Also, convalescent-phase serum samples may be used to identify infectious agents or provide evidence of seroconversion to the agent when paired with acute-phase sera. For standard immunoelectron microscopy, the suspension is incubated for 1 h at 37°C with serum samples diluted in PBS, and then mounted on the grid by using either the drop method or direct centrifugation to the grid. Immunoaggregation may be very powerful in the identification of a suspected or novel agent or with small, dispersed virions (7,13,16). Immuno electron microscopy was particularly useful in the initial identification of noncultivable agents such as hepatitis C, Norwalk virus, and Winnipeg virus (7,13,43). Detailed methods may be found in references (29,32,35,44).
As with all diagnostic laboratory procedures, diagnostic electron microscopy should be performed in a quality-controlled manner. For routine external quality control, the Konsiliarlaboratorium für EM-Erregerdiagnostik at the Robert Koch-Institut in Berlin has conducted an External Quality Assurance-EM Virus Program, which provides panels of specimens containing different agents, since 1994. More than 95 laboratories from 27 countries participated in EQA-EMV 11 during August and September 2001. Each laboratory used its preferred method for preparation (45). A review of results submitted from participating facilities indicated that 27 of 69 laboratories correctly identified all test samples, while an additional 28 successfully identified four of five positive specimens. A trend towards higher success existed among laboratories that used enrichment procedures (35 of 55) when compared with those that were less successful (4 of 14) (p=0.055). However, experience, as defined by years of service and number of samples examined annually, was another important success factor.
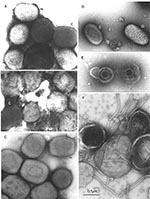
Several major pitfalls exist in the identification of viral agents by negative stain electron microscopy. First, the failure to detect and identify an agent does not mean that it is not there. Second, if you look long enough and hard enough, you will eventually find something that resembles what you wish to find. Third, the presence of a single picture cannot validate the interpretation of morphology. While the diagnostician must not be afraid to find something novel, the finding must be real. One example is the observation of multiple particles with similar morphology. In addition, photographic records must be made for all possible positive identifications and reviewed to confirm the accuracy of the initial diagnosis. Further, when a particle is assigned to a proper virus family, reviewing the case may be necessary to identify the genus or strain. For example, not all samples with orthopoxvirus morphology will be smallpox (Figure 6). While natural infections of variola virus have been eradicated, many other orthopoxvirus continue to be found and identified, e.g., camel-, cow-, monkey-, mouse-, and vaccinia pox viruses (17,46). In addition, the molluscipoxvirus Molluscum contagiosum is morphologically indistinguishable from orthopoxviruses. Identification of Molluscum contagiosum was essentially non-existent in Winnipeg before 1983. With the growth of the immunocompromised sector of the population, the number of identifications increased to 6–10 cases per year until 1995, when the change in sampling methods from lesion aspirates to swab collection in transport medium resulted in a reduction to 1–2 Molluscum contagiosum identifications per year (Hazelton, unpub. data). Further differentiation of poxviruses into variola, vaccinia, cowpoxviruses, or Molluscum contagiosum may be performed by immuno electron microscopy with type-specific antibodies. Appropriate antibodies and the latest nucleic acid amplification techniques are also available for this determination at the World Health Organization Collaborating Centers at the Centers for Disease Control and Prevention, and VECTOR, Koltsovo, Novosibirsk Region, Russia.
Compared with other laboratory diagnostic methods, electron microscopic excels with respect to rapidity and the open view that permits detection and identification of both novel agents and those not considered by the clinician. However, full exploitation of this potential requires early and coordinated application of electron microscopy with other frontline diagnostic procedures. The use of electron microscopy to examine diagnostic cultures of Hendra virus provided evidence of a paramyxovirus 3 days before any other results were available. Thus, focusing further characterization on the proper virus family was possible, and a novel pathogenic agent, which became the prototype strain for the henipah viruses, a proposed new genus of paramyxoviruses, was found (17,47). Diagnostic electron microscopy does not need to be either expensive or difficult to perform if executed in a diagnostic network, i.e., by recruiting instruments and electron microscopists working in other departments, e.g., cell biology or pathology (48). Respective arrangements are facilitated by using inactivated samples and implementing new technologies, such as automated pattern recognition (49) and telemicroscopy by using digital image acquisition and remote operation of the instrument or review of micrographs through the Internet (50).
As smallpox diagnosis from the 1940s to the 1970s, electron microscopy differential diagnosis has often ruled out the occurrence of dangerous pathogens. The power to rapidly identify agents of bioterrorism has now been demonstrated convincingly by Tom Geisbert and Peter Jahrling, U.S. Army Medical Research Institute of Infectious Disease, when they identified and quantified spores in the B. anthracis bioterrorist letter attack upon U.S. Senate Majority Leader Daschle (Jahrling, pers. commun.) (Figure 7). Because the unusual and unexpected can be rapidly identified, electron microscopy must remain a frontline method for rapid diagnostic virology, investigation of potential bioterrorist events, and investigation of new and unusual cases of suspected infectious origin.
Dr. Gelderblom is head of the electron microscope and imaging group and the Konsiliarlaboratorium für EM-Erregerdiagnostik at the Robert Koch-Institut, Berlin, Germany. He is a virologist with over 30 years’ experience in electron microscopic viral diagnostics and an interest in structure-function studies of complex viruses.
Dr. Hazelton is electron microscopist for the department of medical microbiology and infectious diseases, University of Manitoba, Winnipeg, Canada. He is also a virologist with over 20 years’ experience in electron microscopic viral diagnostics. His research interests include viral assembly and transmembrane transport, and the pathogenesis and epidemiology of gastroenteric viruses. He and Dr. Gelderholm have mutual interest in rapid electron microscopic diagnostics and improved detection methods for electron microscopic diagnostics.
Acknowledgments
The authors thank Andrea Männel, Robert Koch-Institut, and the staff of Virus Diagnostic Unit at Cadham Provincial Laboratory for excellent assistance in electron microscopic viral diagnostics; Wolfgang Lorenz for performing the artwork; Bärbel Jungnickl for dedicated darkroom work; F. Bovell, K.M. Coombs, and J. Embree for critical review; and the laboratories listed in Appendix A for their help in establishing an international reference network for rapid diagnostic electron microscopy.
Part of this work was supported by a grant to HG from the European Union, Brussels, (QLK1‑CT‑1999‑00594)
References
- Jackson RJ, Ramsay AR, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75:1205–10. DOIPubMedGoogle Scholar
- Lane HC, LaMontagne J, Fauci AS. Bioterrorism: a clear and present danger. Nat Med. 2001;7:1271–3. DOIPubMedGoogle Scholar
- Hay A. A magic sword or a big itch: an historical look at the United States biological weapons programme. Med Confl Surviv. 1999;15:215–34. DOIPubMedGoogle Scholar
- Wetter DC, Daniell WE, Treser CD. Hospital preparedness for victims of chemical or biological terrorism. Am J Public Health. 2001;91:710–6. DOIPubMedGoogle Scholar
- Lee KE, Umapathi T, Tan C-B, Tjia HT-L, Chua T-S, Oh HM-L, The neurological manifestations of Nipah virus encephalitis, a novel paramyxovirus. Ann Neurol. 1999;46:428–32. DOIPubMedGoogle Scholar
- Hazelton PR, Aoki FY, Hammond GW, Coombs KM, Dawood M. Identification of a proposed novel agent of viral gastroenteritis. 18th Annual Meeting of the American Society for Virology; 1999 Jul 10–14; Amherst, MA. Washington: American Society for Microbiology.
- Gelderblom HR, Hazelton PR. Specimen collection for electron microscopy. Emerg Infect Dis. 2000;6:433–4. DOIPubMedGoogle Scholar
- Krüger DH, Schneck P, Gelderblom HR. Sixty years ago: Helmut Ruska and the visualization of viruses. Lancet. 2000;355:1713–7. DOIPubMedGoogle Scholar
- Nagler FPO, Rake G. The use of electron microscopy in diagnosis of variola, vaccinia and varicella. J Bacteriol. 1948;55:45–51.
- Van Rooyen CE, Scott MA. Smallpox diagnosis with special reference to electron microscopy. Can J Public Health. 1948;39:467–77.PubMedGoogle Scholar
- Brenner S, Horne RW. Negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959;34:103–10. DOIPubMedGoogle Scholar
- Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27nm particle associated with acute infectious non-bacterial gastroenteritis. J Virol. 1972;10:1075–81.PubMedGoogle Scholar
- Madeley CR, Cosgrove BP. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;2:451–2. DOIPubMedGoogle Scholar
- Biel SS, Gelderblom HR. Diagnostic electron microscopy is still a timely and rewarding method. J Clin Virol. 1999;13:105–19. DOIPubMedGoogle Scholar
- Plummer FA, Hammond GW, Forward K, Sekla L, Thompson LM, Jones SE, An erythema infectiosum-like illness caused by human parvovirus infection. N Engl J Med. 1985;313:74–9. DOIPubMedGoogle Scholar
- van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Virus taxonomy: classification and nomenclature of viruses, 7th Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press; 2000.
- Noel JS, Ando T, Leite JP, Green KY, Dingle KE, Estes MK, Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol. 1997;53:372–83. DOIPubMedGoogle Scholar
- Green KY, Belliot G, Taylor JL, Valdesuso J, Lew JF, Kapikian AZ, A predominant role for Norwalk-like viruses as agents of epidemic gastroenteritis in Maryland nursing homes for the elderly. J Infect Dis. 2002;185:133–46. DOIPubMedGoogle Scholar
- Hammond GW, Ahluwalia GS, Klisko B, Hazelton PR. Human rotavirus detection by counterimmunoelectrophoresis versus enzyme immunoassay and electron microscopy after direct ultracentrifugation. J Clin Microbiol. 1984;19:439–41.PubMedGoogle Scholar
- Jiang X, Turf E, Hu J, Barrett E, Dai XM, Monroe S, Outbreaks of gastroenteritis in elderly nursing homes and retirement facilities associated with human caliciviruses. J Med Virol. 1996;50:335–41. DOIPubMedGoogle Scholar
- Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–70.PubMedGoogle Scholar
- Oberste SM, Maher K, Kilpatrick DR, Flemister MR, Brown BA, Pallansch MA. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol. 1999;37:1288–93.PubMedGoogle Scholar
- Sakon N, Yamazaki K, Utagawa E, Okuno Y, Oishi I. Genomic characterization of human astrovirus type 6 Katano virus and the establishment of a rapid and effective reverse transcription-polymerse chain reaction to detect all serotypes of human astrovirus. J Med Virol. 2000;61:125–31. DOIPubMedGoogle Scholar
- Ando T, Monroe SS, Gentsch JR, Jin Q, Lewis DC, Glass RI. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J Clin Microbiol. 1995;33:64–71.PubMedGoogle Scholar
- Vinjè J, Deijl H, van der Heide R, Lewis D, Hedlund K-O, Svensson L, Molecular detection and epidemiology of Sapporo-like viruses. J Clin Microbiol. 2000;38:530–6.PubMedGoogle Scholar
- Gelderblom HR, Asjö B, Moore JP, Ho DD, Sandford J, Pauli G. (1994). Are clinical isolates morphologically different from laboratory strains of HIV? 10th Interntational Conference on AIDS. 1994; Yokohama. AIDS, Abstract PA0195, Vol. 2, Yokohama.
- Kapikian AZ, Feinstone SM, Purcell RH, Wyatt RG, Thornhill TS, Kalica AR, Detection and identification by immune electron microscopy of fastidious agents associated with respiratory illness, acute nonbacterial gastroenteritis, and hepatitis A. Perspect Virol. 1975;9:9–47.
- Miller SE. Diagnosis of viral infections by electron microscopy. In: Lennette EH, Lennette ET, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington: American Public Health Association; 1995. p. 37–78.
- Lew JF, LeBaron CW, Glass RI, Torok T, Griffin PM, Wells JG, Recommendations for collection of laboratory specimens associated with outbreaks of gastroenteritis. MMWR Morb Mortal Wkly Rep. 1990;39(RR-14):1–13.PubMedGoogle Scholar
- Rodgers FG, Hufton P, Kurzawska E, Molloy C, Morgan S. Morphological response of human rotavirus to ultra-violet radiation, heat and disinfectants. J Med Microbiol. 1985;20:123–30. DOIPubMedGoogle Scholar
- Biel SS, Gelderblom HR. Electron microscopy of viruses. In: Cann A, editor. Virus cell culture—a practical approach. Oxford :Oxford University Press; 1999. p. 111–47.
- Hazelton PR, Meyer W, Fischer K, Hannan CK. The effect of electron microscope examination upon the survival of viruses and bacterial spores. Proceedings of the 8th Annual Meeting of the Canadian Society for Biological Safety; 1987 May; Ottawa, Ontario, Canada.
- Long GW, Noble J, Murphy FA, Herrmann KL, Lourie B. Experience with electron microscopy in the differential diagnosis of smallpox. Appl Microbiol. 1970;20:497–504.PubMedGoogle Scholar
- Hayat MA, Miller SE. Negative staining. New York: McGraw Hill; 1990.
- Gelderblom HR, Renz H, Özel M. Negative staining in diagnostic virology. Micron and Micropica Acta. 1991;22:435–47. DOIGoogle Scholar
- Aebi U, Pollard TD. A glow discharge unit to render electron microscope grids and other surfaces hydrophilic. J Electron Microsc (Tokyo). 1987;7:29–33.PubMedGoogle Scholar
- Lewis DC. Three serotypes of Norwalk-like virus demonstrated by solid-phase immune electron microscopy. J Med Virol. 1990;30:77–81. DOIPubMedGoogle Scholar
- Anderson N, Doane FW. Specific identification of enteroviruses by immuno-electron microscopy using a serum-in-agar diffusion method. Can J Microbiol. 1973;19:585–9. DOIPubMedGoogle Scholar
- Hammond GW, Hazelton PR, Chuang I, Klisko B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J Clin Microbiol. 1981;14:210–21.PubMedGoogle Scholar
- Jansons J, Harnett GB, Bucens MR. Electron microscopy after direct ultracentrifugation. Pathology. 1985;17:29–30. DOIPubMedGoogle Scholar
- Gelderblom H, Reupke H. Rapid viral diagnosis using the Airfuge. IV International Congress on Virology, The Hague. Abstract #W50A/9, p. 630.
- Abe K, Kurata T, Shidata T. Non-A, non-B hepatitis: visualization of virus-like particles from chimpanzee and human sera. Arch Virol. 1989;104:351–5. DOIPubMedGoogle Scholar
- Doane FW, Anderson N. Electron microscopy in diagnostic virology. New York: Cambridge University Press; 1987.
- Gelderblom HR. Electron microscopy in diagnostic virology. BIOforum International. 2001;5:64–7.
- Fenner F, Henderson DA, Arita I, Jezek J, Ladnyi LD. Smallpox and its eradication. Geneva: World Health Organization; 1988.
- Murray K, Selleck P, Hooper P, Hyatt A, Gold A, Gleeson L, Morbillivirus that causes fatal disease in horses and humans. Science. 1995;268:94–7. DOIPubMedGoogle Scholar
- Biel SS, Madeley D. Diagnostic virology—the need for electron microscopy: a discussion paper. J Clin Virol. 2001;22:1–9. DOIPubMedGoogle Scholar
- Utagawa ET, Nakazawa E, Matsuo K, Oishi I, Takeda N, Miyamura T. Application of an automated specimen search system installed in a transmission electron microscope for the detection of caliciviruses in clinical specimens. J Virol Methods. 2002;100:49–56. DOIPubMedGoogle Scholar
- Schroeder JA, Voekl E, Hofstaedter F. Ultrastructural telepathology—remote EM diagnostic via internet. Ultrastruct Pathol. 2001;25:301–7. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 9, Number 3—March 2003
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Paul R. Hazelton, Department of Medical Microbiology, 531 Basic Medical Sciences, 730 William Avenue, Winnipeg, MB R3E 0W3, Canada; fax: 204-789-3926
Top