Volume 29, Number 1—January 2023
Dispatch
Photobacterium damselae subspecies damselae Pneumonia in Dead, Stranded Bottlenose Dolphin, Eastern Mediterranean Sea
Abstract
Photobacterium damselae subspecies damselae, an abundant, generalist marine pathogen, has been reported in various cetaceans worldwide. We report a bottlenose dolphin in the eastern Mediterranean Sea that was found stranded and dead. The dolphin had a severe case of chronic suppurative pneumonia and splenic lymphoid depletion caused by this pathogen.
The common bottlenose dolphin (Tursiops truncatus) is perhaps the most common and widespread dolphin species in the Mediterranean Sea (1). Photobacterium damselae subspecies damselae is a pathogen that produces wound infections and hemorrhagic septicemia and high mortality rates and affects various marine animals, such as fish, mollusks, crustaceans, and cetaceans (2,3). Highly pathogenic P. damselae subsp. damselae isolates have 2 major virulence factors: the phospholipase D damselysin (Dly) and the pore-forming toxin phobalysin P (initially called HlyApl). Both toxins are encoded by the plasmid pPHDD1 and produce hemolytic and cytolytic activities in a synergistic manner (4). We report a bottlenose dolphin in the eastern Mediterranean Sea that was found stranded, dead, and had a severe case of chronic suppurative pneumonia and splenic lymphoid depletion caused by this pathogen.
On January 29, 2021, a bottlenose dolphin was found beached nearby Ashdod, Israel. The carcass underwent a postmortem examination based on a widely accepted protocol (5) with some modifications because the carcass was also sampled for several anatomic and physiologic studies. Samples of the spleen, liver, lung, kidney and brain were collected for quantitative PCR molecular detection of Toxoplasma gondii (6) and canine distemper virus (7), and for PCR detection of Brucella spp. (8). Samples of spleen and lung were fixed in 10% buffered formalin for routine histologic evaluation. Samples of lungs and fluid from the thoracic cavity were obtained by using sterile swabs for lung samples and sterile syringes and needles for fluid samples and inoculated onto tryptone soy agar, blood agar (5% sheep blood enriched tryptone soy agar), and MacConkey agar, and incubated for 24–48 h at 37°C. Confirmation of bacteria species was initially performed by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry according to the manufacturer’s protocol (Autoflex; Bruker, https://www.bruker.com).
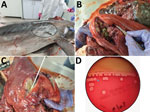
The dolphin weighed 200 kg, had a length of 263 cm, and was identified as a mature female that had a moderate nutritional status (9). At external examination, a deep bruise was observed on the front of the dorsal fin, and an old visible scar was observed on the right side of the chest, which might have been the result of an injury by a foreign body that might have instigated the inflammation within the lung, leading to pneumonia (Figure 1, panel A). No additional external signs of interaction with fishing gear were observed. The carcass was at stage 3 on the decomposition condition code scale (5). Internal examination indicated 4 large, firm nodules, 5–10 cm in diameter, replacing the cranial aspect of the right lung lobe. On cut sections, nodules were filled with purulent to caseous, thick, granular, green-tinged exudate surrounded by a dense fibrous capsule (abscess) (Figure 1, panels B, C). No other abnormalities were observed in all other internal organs.
Pure bacterial colonies of spherical or ovoid cocci, 1–2 μm in diameter, consistent with the genus Photobacterium, appeared on the blood agar plates at 48-hours postinoculation. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry confirmed the initial identification of Photobacterium damselae. The isolate was resistant to ampicillin and susceptible to gentamicin, sulfamethoxazole/trimethoprim, florfenicol, amikacin, and polymyxin B. The isolate also had intermediate susceptibility to amoxicillin/clavulanic acid; fluoroquinolones; and first-, second-, and third-generation cephalosporins.
The isolate species was also characterized and confirmed by using 16S rRNA gene primers and Sanger sequencing of the 800-nt PCR product. Whole-genome sequencing (WGS) was performed to obtain the allelic multilocus sequence typing (MLST) profile for sequence type determination and to analyze the presence of the 2 P. damselae subsp. damselae major virulence factor genes (dly and hlyApl).
We extracted DNA by using the QIAsymphony SP System and the QIAsymphony DNA Mini Kit (QIAGEN, https://www.qiagen.com), according to the manufacturer’s recommendations. We prepared a DNA library by using the Nextera XT Library Preparation Kit (Illumina, https://www.illumina.com), followed by WGS using the Illumina MiSeq and a 250-bp paired-end read length. Reads were assembled by using the BioNumerics 8.0 Platform SPAdes 3.13.1 (Applied Maths, https://www.applied-maths.com).
The assembly was deposited to the pubMLST P. damselae database under identification no. 91. We obtained the allelic MLST profile by using the BioNumerics Sequence Extraction Tool (Applied Maths) and according to the P. damselae scheme based on 6 housekeeping genes (glpF, gyrB, metG, pntA, pyrC, and toxR) (10). This tool was also used for identification of virulence factor gene sequences dly (GenBank accession no. 9937366) and hlyApl (GenBank accession no. ID 9937197). Hemolysis was tested by culturing the isolate on 5% sheep blood agar (#PD-005; Hylabs Ltd, https://www.hylabs.co.il) for 24 h at 37°C.
Identification of P. damselae subsp. damselae was supported and confirmed by molecular, phenotypic, and genomic characterization. The 16S rRNA sequence showed a similarity of 99.17% with other P. damselae subsp. damselae strains in GenBank. When tested for hemolysis, the isolate exhibited a weak hemolytic phenotype, producing narrow halos on sheep blood agar plate (Figure 1, panel D). This phenotype is typical of P. damselae subsp. damselae lacking the pPHDD1 plasmid and having the chromosomal PhlyC gene (hlyAch). WGS of the hemolytic genes dly and hlyApl yielded only the hlyA sequence, which showed 99% identity to the hlyAch sequences in GenBank.
The MLST allelic scheme extraction (Table 1) resulted in a new profile that was submitted to the isolate collection of the PubMLST P. damselae database as PDIN1, and was assigned a new sequence type (ST), ST63. Within the PubMLST database, most of the P. damselae subsp. damselae isolates (Table 2) originated from an unusual cetacean mortality event in Italy during 2013 (11). Neighbor-joining phylogenetic analysis suggested that the strain from Israel sequenced in this study was not strongly related to any other available ST and showed closest resemblance to isolate ST45 from a bottlenose dolphin from Italy (Appendix Figure).
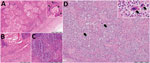
Results of molecular detection for T. gondii, canine distemper virus, and Brucella spp. were negative for all tested samples. Examination of lung tissue (Figure 2, panels A–C) showed a nodular structure covered by fibrous tissue composed of extensive cellular infiltration, numerous cholesterol clefts, and areas of reactive fibrosis. A second section of the lung showed extensive tissue lysis and concentric fibrosis of blood vessels. In part of the section, a locally extensive cellular infiltration was observed. An area of necrosis was accompanied by a neutrophilic inflammatory reaction and intralesional bacterial colonies. Two additional tissue sections showed diffuse solid fibrosis, multiple cholesterol clefts, and aggregations of leukocytes. Histopathologic analysis indicated an apparent contraction of the parenchyma with occasional lymphoid follicles and diffuse cellularity within the spleen (Figure 2, panel D), which were suggestive of extramedullary hematopoiesis. Morphologic features of both organs included severe chronic suppurative pneumonia and splenic lymphoid depletion, possibly resulting in extramedullary hematopoiesis in the spleen.
This strain caused severe chronic suppurative pneumonia in the absence of the dly gene. This result supports previous indications that this virulence factor is not essential for pathogenesis (12).
The antibacterial drug sensitivity test showed susceptibility of the isolate to drugs most frequently used in human and veterinary medicine in this region. Tests results for T. gondii, canine distemper virus, and Brucella spp. showed negative results, making P. damselae subsp. damselae the only culturable pathogen identified in the dolphin.
We report detection of P. damselae subsp. damselae in a bottlenose dolphin in the Mediterranean Sea. This report adds to the increasing baseline data regarding the health of these marine mammals and provides molecular information for a pathogen capable of infecting a large variety of animals in the marine environment, as well as humans.
Dr. Morick is a veterinarian, researcher, and head of the marine pathology laboratory at the Morris Kahn Marine Research Station, Haifa, Israel. His primary research interests are marine animals, pathogen emergence, disease transmission, aquatic animals, marine biology, marine ecology, and public health.
Acknowledgments
This study was supported by the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China (grant SMSEGL20SC02) and by the Kahn Foundation.
D.M., N.D., E.B., Y.Z., A.S., and K.A. contributed to field collections, necropsy procedure, and sample processing; Z.Z.S., A.R., I.N., D.T., N.W., P.I., and M.R.E. contributed to data processing, pathologic interpretation, and writing of the manuscript; and I.N., N.F., A.R., S.B., and M.F. performed bacterial isolation and molecular characterization. All authors participated in drafting the manuscript, contributed to writing the article, and approved the submitted version.
References
- Bearzi G, Fortuna CM, Reeves RR. Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea. Mammal Rev. 2009;39:92–123. DOIGoogle Scholar
- Rivas AJ, Lemos ML, Osorio CR. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol. 2013;4:283. DOIPubMedGoogle Scholar
- Osorio CR, Vences A, Matanza XM, Terceti MS. Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J Bacteriol. 2018;200:e00002–00018. DOIPubMedGoogle Scholar
- Rivas AJ, Balado M, Lemos ML, Osorio CR. The Photobacterium damselae subsp. damselae hemolysins damselysin and HlyA are encoded within a new virulence plasmid. Infect Immun. 2011;79:4617–27. DOIPubMedGoogle Scholar
- Geraci JR, Lounsbury VJ. Marine mammals ashore: a field guide for strandings. 2nd ed. National Aquarium in Baltimore. College Station (TX): Texas A&M University Press; 2005.
- Bigal E, Morick D, Scheinin AP, Salant H, Berkowitz A, King R, et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus); A first description from the Eastern Mediterranean Sea. Vet Parasitol. 2018;258:74–8. DOIPubMedGoogle Scholar
- Elia G, Decaro N, Martella V, Cirone F, Lucente MS, Lorusso E, et al. Detection of canine distemper virus in dogs by real-time RT-PCR. J Virol Methods. 2006;136:171–6. DOIPubMedGoogle Scholar
- Bardenstein S, Waner T, Etinger M, Even Tof B, Blum S, Bellaiche M, et al. First diagnosis of Brucella canis infection in dogs in Israel. Isr J Vet Med. 2021;76:12–8.
- Sharir Y, Kerem D, Gol’din P, Spanier E. Small size in the common bottlenose dolphin Tursiops truncatus in the eastern Mediterranean: a possible case of Levantine nanism. Mar Ecol Prog Ser. 2011;438:241–51. DOIGoogle Scholar
- Alba P, Caprioli A, Cocumelli C, Ianzano A, Donati V, Scholl F, et al. A new multilocus sequence typing scheme and its application for the characterization of Photobacterium damselae subsp. damselae associated with mortality in cetaceans. Front Microbiol. 2016;7:1656. DOIPubMedGoogle Scholar
- Casalone C, Mazzariol S, Pautasso A, Di Guardo G, Di Nocera F, Lucifora G, et al. Cetacean strandings in Italy: an unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis Aquat Organ. 2014;109:81–6. DOIPubMedGoogle Scholar
- Osorio CR, Romalde JL, Barja JL, Toranzo AE. Presence of phospholipase-D (dly) gene coding for damselysin production is not a pre-requisite for pathogenicity in Photobacterium damselae subsp. damselae. Microb Pathog. 2000;28:119–26. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: December 18, 2022
Table of Contents – Volume 29, Number 1—January 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Danny Morick, Morris Kahn Marine Research Station, University of Haifa, Haifa 3498838, Israel
Top